With annual crops, the time of greatest risk is likely to be early in a growth season, and especially during seedling establishment. Chilling injury to seedlings can show up as necrotic lesions on the young roots and shoots, with slowed growth and increased susceptibility to disease attack, and even death. Crops adversely affected by low temperatures during the establishment period take longer to mature and this in turn may mean that they are at risk to chilling temperatures towards the end of the growing season.
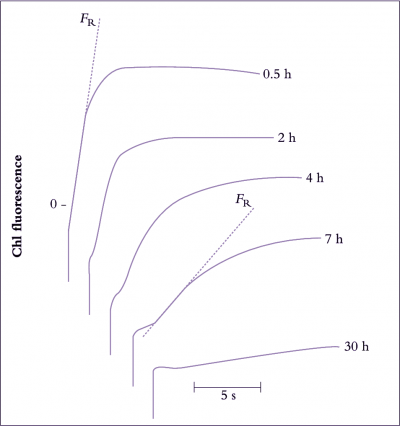
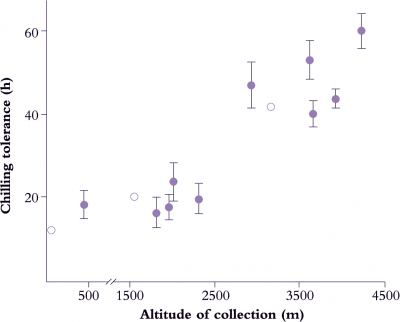
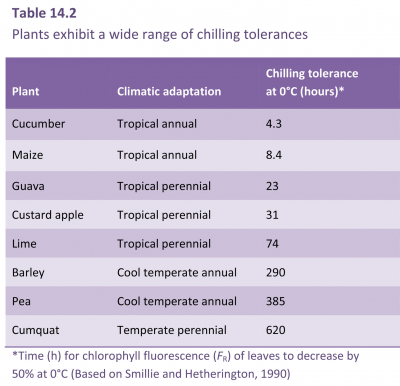
Not only does chilling exposure retard the growth and maturation of crops, but chilling damage to fresh produce during postharvest storage is also of economic importance (Figure 14.17, and see Section 11.6.5). Chilling injury is a particular problem with fresh fruit, vegetables and flowers, because storage at temperatures low enough to retard tissue respiration is still the most effective postharvest method for extending the shelf life of produce. Even in produce-handling industries, there is often insufficient appreciation of requirements and behaviour of individual crops or even specific cultivars, and losses ensue. The time taken for symptoms to develop varies greatly and is influenced by a number of factors including genotype, cultivar, stage of maturity and preharvest growth conditions. For example, with fruit stored at 1–2°C, it takes several months for chilling injury to develop in apples as a brown discolouration of the cortex, several weeks for the flesh of peaches to become mealy in texture, a number of days for avocados to show areas of grey discolouration in the flesh, and only a few hours for cucumbers to display tissue breakdown in the mesocarp. Obviously storage at 0–2°C is an excellent method for extending the storage life of apples, is moderately useful for peaches, but disastrous for cucumbers. Avocados are better kept at a higher storage temperature; the recommendation for extended storage of avocados is 6°C. Even this temperature is too low for tomato, another sub-tropical species susceptible to chilling injury. Ripe tomatoes should be stored cool, at 12°C or above, and not at refrigerator temperature.
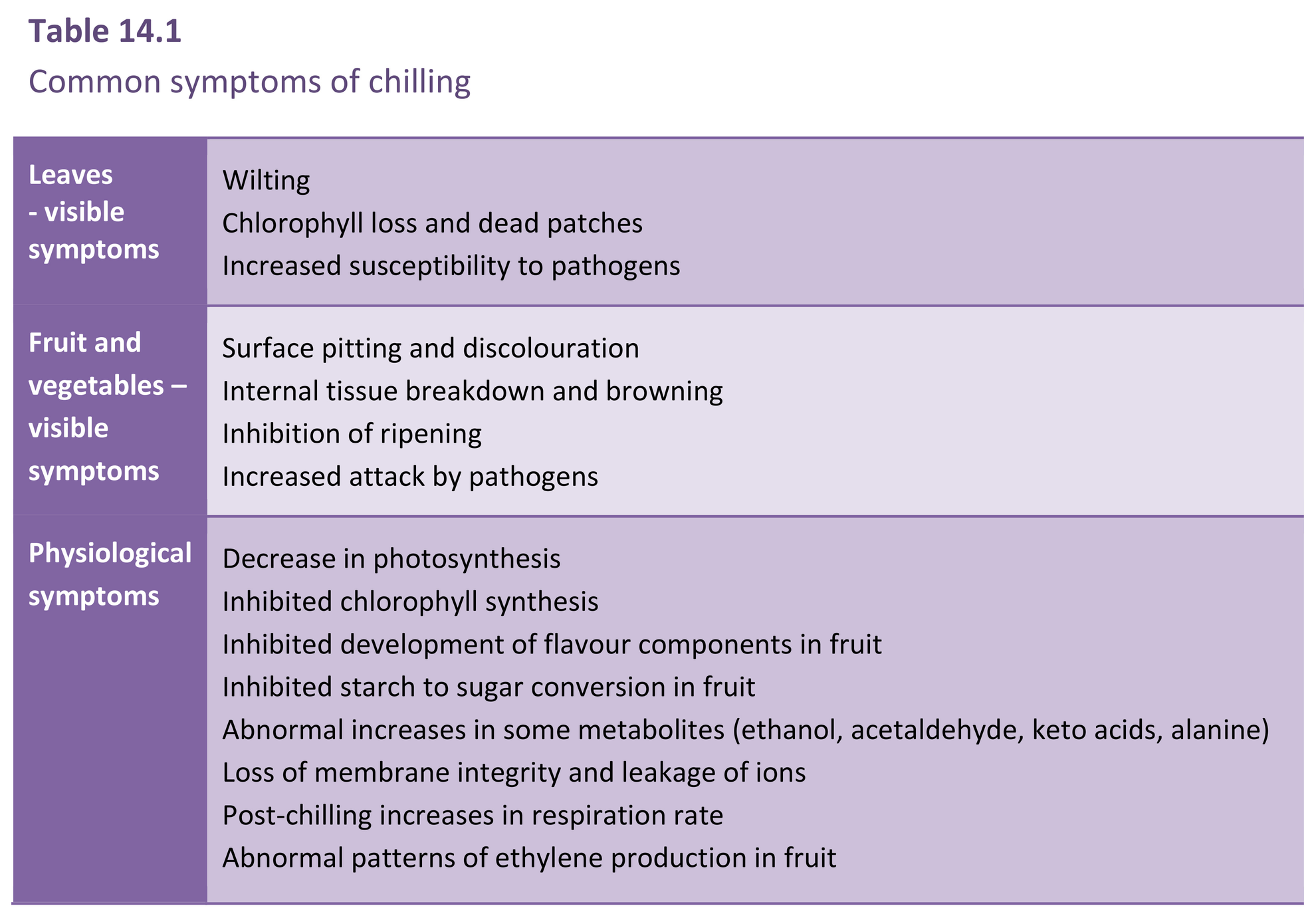
Chilling injury becomes apparent in a variety of ways (Table 14.1) that vary with species and tissue. Visible symptoms are outcomes of physiological disorders, and may develop slowly during the actual chilling period, to be expressed more rapidly once the tissue is returned to warmer, non-chilling conditions.
In defining the physiological basis of chilling injury, loss of membrane integrity emerges as a major symptom and much research has been directed to elucidating the chemical and physical nature of lipoprotein membranes of species having different climatic origins. Dr John Raison and other scientists at the former CSIRO Division of Food Research in Sydney provided evidence that physical changes occur in membranes of chilling-susceptible plants during low-temperature exposure. They suggested that the molecular ordering of membrane lipids is altered in the temperature range where chilling effects become apparent. In particular, lipid composition appears to determine how membranes respond to low temperatures. Tropical species tend to have lipids with a higher proportion of saturated fatty acids (these are fatty acids such as palmitic acid which lack double bonds in their structure and therefore have higher melting points), while cool-climate plants tend to have more unsaturated fatty acids such as oleic acid. However, a consistent pattern of differences in lipid membrane composition between chilling-susceptible and chilling-resistant plants has yet to emerge and additional factors are likely to be involved. The physical nature of cell membranes remains an important point for research into chilling injury, but as yet no single physiological factor has been linked with plant susceptibility to chilling injury.