14.7 - High temperature stress
Aidan D Farrell, University of the West Indies, Trinidad
Exposure to excessive temperatures during development limits the yield of many of the world’s major crops, especially in the tropics. Increasing global temperatures over the last three decades have resulted in significantly reduced yields in many crops. In addition to the general warming, a predicted increase in the occurrence of heatwaves is likely to result in further yield losses (Long and Ort 2010). Increasing global temperatures and increasingly frequent heatwaves are likely to have similarly negative effects on natural systems in the tropics and subtropics.
In daylight hours leaf temperatures are often higher than that of the surrounding air, as the canopy absorbs incident solar radiation. Overheating occurs when heat dissipation from the canopy is unable to keep pace with the thermal energy absorbed (for plant energy budget see Section 14.1.3). This typically occurs when incident radiation is high and transpirational cooling is low. Even at temperate latitudes, such conditions often develop at midday when solar radiation peaks and soil water reserves are depleted. In warm, dry environments heat stress can persist for prolonged periods. As heat stress is frequently encountered in combination with water deficit and excess irradiance, it can be difficult to disentangle the effects of the three factors. Nonetheless, there is a distinct set of injuries and plant responses that are associated with heat stress. These are detailed in the following sections.
The effect of heat stress on staple crops like wheat can be severe. The impact varies depending on the developmental stage of the plants, with the most vulnerable stage being flowering. High temperatures shorten the duration of growth of both the leaves and the grains, accelerating their development and thus limiting the ability of the plant to accumulate the carbohydrate necessary for grain growth. In addidtion, heat stress before flowering can cause floret sterility, causing yield losses due to reduced grain number. This effect is most acute when heat occurs at or just after pollen meiosis, when carbohydrate supply to the developing pollen grains appears most critical. Grain size in heat stressed plants can be severely reduced, predominately from a reduction in starch, which makes up most of the mass of the grain.
Wheat (a temperate C3 species) produces its most grain at temperatures below 26°C; and its yield is reduced at higher temperatures. Yet in most grain-growing areas in the southern hemisphere, and in Meditteranean climates in the northern hemisphere, temperatures increase steadily during the growing season, and brief periods above 30 °C often occur during the grain-filling period. In many arid countries mean day temperatures can easily exceed wheat’s high temperature threshold during much of the growing season and so heat stress can significantly reduce crop yield by accelerating plant senescence, diminishing seed number and final seed weight.
Where vegetation is sparse, maximum daytime temperatures occur at the soil surface where exposed soil absorbs solar radiation and quickly warms above ambient. Under such conditions soil temperatures can exceed 50oC. Exposed soil is a particular problem when planting crops in warm regions where the dark, moist soil surface can reach high temperatures and severely inhibit germination and seedling emergence (http://www.plantstress.com/Articles/heat_i/heat_i.htm). A similar phenomenon has been seen in temperate climates when plastic mulch is used to artificially insulate the soil surface during planting (Farrell and Gilliland 2011).
14.7.1 - Plant response to high temperature
Plants have developed a range of mechanisms to keep tissues from overheating (heat avoidance) or to prevent inhibition and injury where high temperatures occur (heat tolerance). A key aspect of tolerance to heat stress is the degree to which a tissue exposed to moderately high temperatures can acclimate in a way that improves its ability to function at higher temperatures (hardening or acquired thermotolerance).
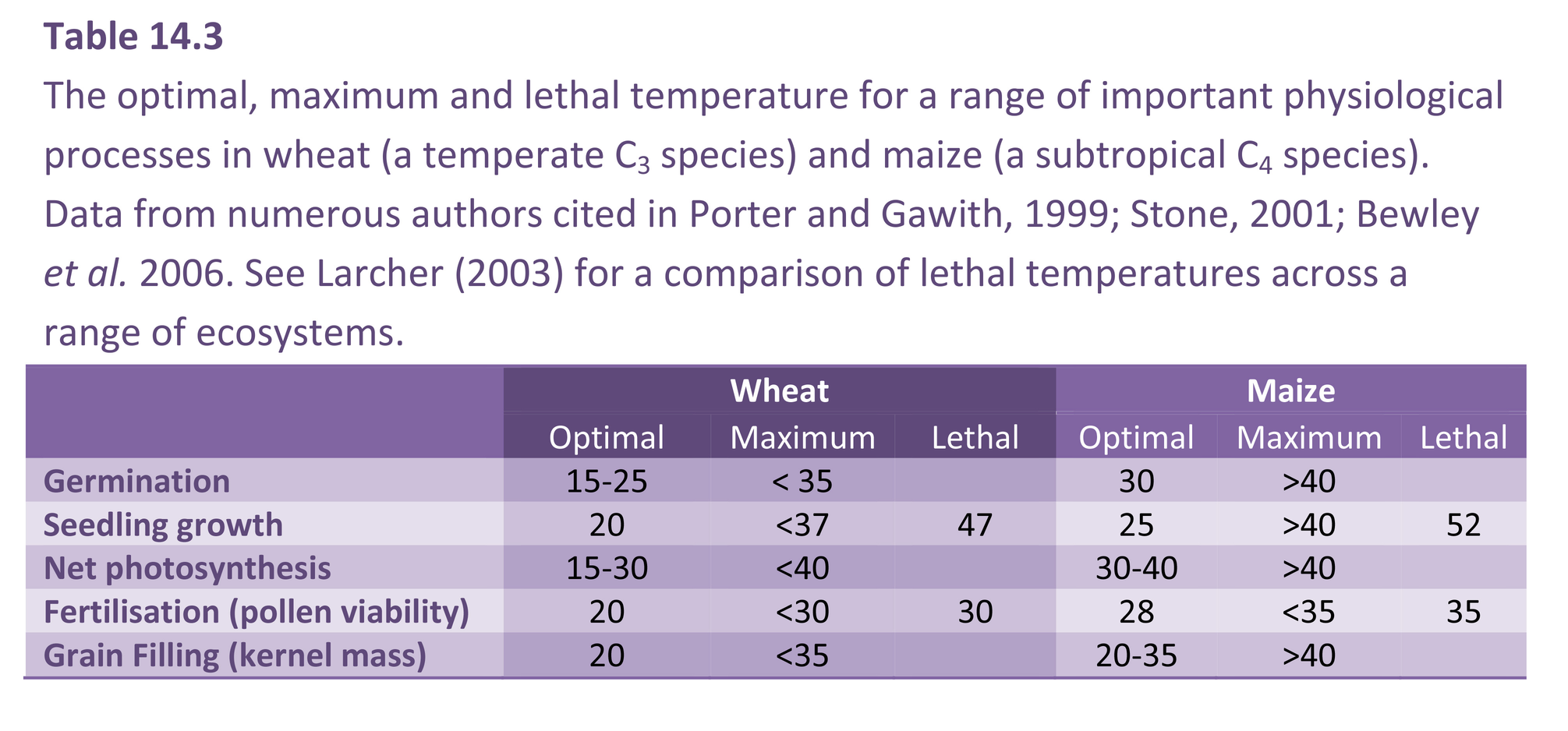
Table 14.3 shows the optimum, maximum and lethal temperature for a range of processes in wheat and maize. Optimum temperatures for plant metabolism vary between species, reflecting the thermal range found in the environment in which they evolved or were selected (http://www.plantstress.com/Articles/heat_i/heat_i.htm). Metabolic processes in plants typically have an optimum below 30oC and temperatures above 40oC are considered a stress. The degree of damage from heat stress is mediated by the intensity, duration and rate of change in temperature as well as by the plant’s developmental stage and growing conditions prior to exposure (Larkindale et al. 2005; Allakhverdiev et al. 2008; Mittler et al. 2012). For most species, actively growing tissue is damaged by brief exposure to temperatures above 45oC, while prolonged exposure can result in fatal injury. Temperatures between 30-40oC can be termed moderately high temperatures and result in reversible inhibition of metabolism (moderate heat stress). Temperatures above 40oC can be termed very high temperatures as they result in irreversible or prolonged inhibition of metabolism (severe heat stress).
The effect of heat stress is often measured by exposing tissue to high temperatures for a short period (typically 0.5-1 hour) and measuring the response. This can be termed high temperature shock (heat shock). In addition to identifying the maximum threshold temperature above which metabolic activity ceases, heat shock experiments can determine the lethal temperature above which irreversible injury occurs (Table 14.3). Although lethal temperatures rarely occur when plants are grown within their native range, determining the lethal temperature is a useful method for assessing a species tolerance to high temperature in general. Species adapted and/or acclimated to high temperature environments can withstand temperatures well above 40oC. Extreme examples of tolerance to high temperature are found in the desert succulents, such as the prickly pear cacti (Opuntia spp.) which can survive short term exposure to 70oC (Nobel, 1988).
14.7.2 - Heat avoidance
Fig14.27.jpg
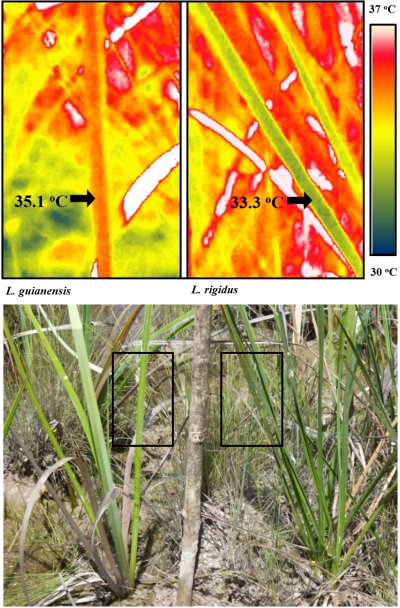
Figure 14.27. Thermal images showing midday leaf surface temperature of two savanna sedges, Lagenocarpus guianensis (left) and Lagenocarpus rigidus (right), growing in close proximity at a forest edge. The accompanying photograph indicates the areas used for the thermal images. The white areas in the thermal image represent dead leaves which are above the maximum temperature range. Replicated measurements showed the midday leaf surface temperatures were significantly different (P <0.001) between the two species. The greater potential for ‘canopy temperature depression’ allows L. rigidus to grow in the open savanna, while L. guianensis finds its range restricted to the shaded savanna edges where heat and light are less overbearing (see John-Bejai et al. 2013 http://aobpla.oxfordjournals.org/content/5/plt051.full).
Plants can avoid overheating by regulating the components of their energy budget. The amount of solar radiation intercepted can be reduced using specialised leaf and canopy arrangements. The vertical leaves and canopy architecture of many eucalyptus trees are arranged to minimise the area of the canopy exposed to direct sun (as a consequence they cast a relatively small shadow). Other species, such as dragon trees (Dracaena spp.) and some acacia trees, adopt an umbrella-like form to raise the canopy above the warm ground and shade the trunk. Some species employ leaf movements, rolling their leaves or changing leaf orientation so that the surfaces are never parallel to the sun. Leaf movements are particularly common in legumes (Fabaceae).
The amount of incident radiation absorbed by a leaf can be reduced by increasing the reflectance of the leaf surface. Leaf hairs and scales scatter the incident radiation such that the leaf appears silvery white, e.g. the sagebrush (Artemisia tridentata) and the brittlebush (Encelia farinosa) common in many arid areas of North America. Pubescence is common in hot, dry environments, where as well as reflecting solar radiation, leaf hairs form a thick boundary layer reducing water loss (although this also reduces the potential for transpirational cooling). Such adaptations are so common among plants of warm arid and high altitude regions that these habitats can be seen to form a ‘silvery landscape’. Waxes deposited on the epidermis perform a similar function, preventing water loss and forming an irregular surface to increase reflectance.
The amount of heat lost can also be regulated by favouring small or divided leaves that reduce the boundary layer allowing for greater convective cooling as well as more effective transpiration. Tropical savanna plants with smaller leaves are better able to keep cool in the full glare of the equatorial sun, while species with larger leaves are restricted to areas shaded by tree canopies (Figure 14.27). Small leaves are also a distinguishing feature of desert shrubs and trees.
An increase in air temperature reduces the relative humidity (increases the vapour pressure deficit), which increases the evaporative demand and the transpiration rate. Where water supply is restricted the stomata will close causing transpiration rate to fall, resulting in an increase in leaf temperature. Where water supply is not limiting, transpirational cooling is an effective form of heat avoidance. Transpirational cooling often reduces leaf temperatures to 5oC below ambient, while temperatures can be reduced by 15oC in extreme cases. This contrasts with plants growing in arid conditions where leaf temperatures may be 15oC or more above ambient. In agricultural environments, where high temperatures are not necessarily combined with water deficit, cultivars showing high stomatal conductance have been shown to be more resistant to heat stress. In fact, stomatal conductance measurements along with direct measurements of ‘canopy temperature depression’ are among the most valuable parameters for selecting cultivars for growth in warm environments. The recent application of infrared thermometers to examine canopy temperatures has provided a valuable method for directly measuring heat avoidance (Figure 14.27); http://www.plantstress.com/Articles/heat_m/heat_m.htm; http://www.plantstress.com/methods/IRT_protocol.htm).
14.7.3 - Heat injury and inhibition
Heat stress affects plants through three principle mechanisms: excessive membrane fluidity; disruption of protein function and turnover; and metabolic imbalances. Metabolic imbalances can be due to differences in the activation energy of the component reactions, or to the effect of the other two mechanisms on the thermal response of each reaction. The inhibition of metabolism from these three mechanisms also results in the accumulation of toxic compounds and reactive oxygen species (ROS), the removal of which is also inhibited by heat stress.
(a) Whole plant effects
Generally, inhibition of photosynthesis is seen as a critical factor in heat stress. Net photosynthesis is typically the first process to be inhibited at high temperatures (Berry and Bjorkman 1980; Allakhverdiev et al. 2008). As temperature rises above optimum, gross photosynthesis is inhibited while respiration and photorespiration increase. The combined effect of these three processes is a marked reduction in net photosynthesis during moderate heat stress (Figure 14.12).
C4 plants do not suffer from the increase in photorespiration and so can maintain a higher photosynthetic optimum; however, the maximum temperature does not vary to the same extent. The imbalance between photosynthesis and respiration is itself damaging, as carbohydrate reserves can become depleted. As temperature rises further, membrane transport and respiration become inhibited, eventually leading to cell death. Both the light reactions and the Calvin cycle are highly sensitive to moderate heat stress. Injury following severe heat stress is perhaps most acute for the light reactions, with even brief exposure resulting in long-term inhibition of photosystem II (PSII). As the activity of PSII is highly temperature sensitive it can be used as an indicator of heat stress and heat injury; measurements of chlorophyll fluorescence have been widely used for this purpose (http://prometheuswiki.publish.csiro.au/tiki-index.php?page=Chlorophyll+fluorescence).
For many years, the inhibition of gross photosynthesis was thought to occur at temperatures too low to be explained by the thermal deactivation of photosynthetic enzymes. Experiments comparing the thermal response of many steps in the photosynthetic apparatus, suggested the initial inhibition was due to the sensitivity of the thylakoid membrane to high temperatures (Berry and Bjorkman 1980). However, this view has been questioned recently with the observation that at moderately high temperatures photosynthetic inhibition coincides with a reversible reduction in the activity of certain Calvin cycle enzymes (Sharkey 2005). Severe heat stress is still thought to be due to injury of PSII, through direct cleavage of the D1 protein and a range of other mechanisms. Although the thermal sensitivity of PSII is not solely due to the thermal sensitivity of cell membranes, membrane properties are a major regulator of both inhibition and injury of PSII (Sharkey 2005; Allakhverdiev et al. 2008).
The thermal sensitivity of reproductive processes can be a limiting factor for plant productivity and it is often the critical factor for crop production in areas prone to heat stress (Table 14.3; http://www.plantstress.com/Articles/heat_i/heat_i.htm). Heat stress can reduce the duration of reproductive development and severely inhibits floral development, fertilization and post fertilization processes in many species. Pollen viability is particularly vulnerable to heat damage. Severe heat stress inhibits both the photosynthetic source and the reproductive sink, resulting in a significant reduction in the number and size of seeds and/or fruit. This is a particular problem in fruit and grain crops such as tomato, cowpea, wheat, and maize (http://www.plantstress.com/Articles/heat_i/heat_i.htm).
At high temperatures dry matter production is often more limited by photosynthesis than by cell expansion (while at low temperatures dry matter production is more limited by cell expansion than by photosynthesis). Generally, the inhibition of photosynthesis and other growth maintaining processes during moderate or short-term heat stress results in a comparatively small reduction in the rate of dry matter production (relative growth rate) (Chapter 6.2.2; http://www.plantstress.com/Articles/heat_i/heat_i.htm). As temperature increases within a plant’s thermal range, the duration of growth decreases but the rate of growth increases, as shown earlier in this chapter. As a consequence, organ size at maturity may change very little in response to temperature, despite variation in growth rate. As temperatures are raised further, an increased rate of growth is no longer able to compensate for a reduction in the duration of development, and the final mass of any given organ at maturity is reduced. This response can be seen in a range of tissues including leaves, stems and fruit. A smaller organ size at maturity due to high temperature is associated with smaller cells rather than a change in cell number. This implies that cell enlargement is more sensitive to temperature than is cell division. The reduced duration of development can also limit the number of organs that are produced, e.g. grain number in wheat is reduced when plants are grown at moderately high temperatures (Stone and Nicolas 1994). Under certain conditions plants grown under moderate heat stress accumulate sugars in their leaves, indicating that translocation can be more limiting than photosynthesis, but this is not thought to be a general limitation.
(b) Membrane properties
The structure and fluidity of lipid membranes is dependent on their composition and on temperature. An increase in temperature will result in an increase in the fluidity of lipid membranes as the hydrogen bonding between adjacent fatty acids become weak. This increase in fluidity is associated with an uncontrolled increase in membrane permeability as the activity of membrane bound proteins is disrupted. Indeed, this uncontrolled membrane permeability is used as an assay to test for damage due to heat stress (http://www.plantstress.com/Methods/CMS_method.htm).
Membrane-associated processes, such as photosynthesis and membrane transport, are typically the first to be inhibited during exposure to high temperature (Berry and Bjorkman 1980; Allakhverdiev et al. 2008). The high temperature sensitivity of PSII is thought to be due, at least in part, to its close association with the thylakoid membrane. In addition to these direct effects on metabolic function the changes in membrane fluidity during heat stress act as a signal to initiate other stress responses in the cell (Mittler et al. 2012).
(c) Protein function and turnover
For most metabolic reactions, the optimum and maximum temperatures are determined by the thermal response of key enzymes. Enzymes act to lower the activation energy and increase the rate of reactions at any given temperature. However, as temperature increases the catalytic properties of most enzymes are lost and they begin to denature (i.e. enzymes are thermolabile). The synthesis of replacement enzymes and other cell proteins is also impaired, resulting in an overall limitation due to reduced protein turnover. Under prolonged severe heat stress many enzymes will become denatured. This, combined with the loss of membrane function will result in cell death.
For some reactions, the thermal response of a particular enzyme can be rate limiting. The inhibition of photosynthesis during moderate heat stress has been associated with a reduction in the catalytic activity of Rubisco (Ribulose 1:5 bisphosphate carboxylase/oxygenase), due in part to the thermal sensitivity of Rubisco activase. In some species, production of heat stable forms of Rubisco activase has been shown to play role in acclimation to high temperature (Yamori et al. 2013). There have been attempts to engineer less temperature sensitive forms of Rubisco activase in order to increase the thermal range of crop species, but it remains to be seen if altering a single component of the photosynthetic system will improve overall heat tolerance (Sharkey 2005; Allakhverdiev et al. 2008).
(d) Metabolic imbalances
When a plant is grown outside of its optimum thermal range, metabolic imbalances occur. Imbalances may result in a short-fall of essential metabolites or intermediaries, or in a build-up of substances that becomes toxic (e.g. aggregated proteins). Such imbalances cause further inhibition of processes such as photosynthesis and respiration. The imbalances can be due to differences in the thermal response of particular reactions. For instance, the enzymes used in photosynthesis are deactivated at a lower temperature than those used in respiration. This has the result that as temperatures increase, the rate of carbon fixation falls while the rate of carbon use may rise. The point at which the plant is using more carbon than it is assimilating is termed the ‘temperature compensation point’. Beyond the temperature compensation point, the plant begins to use up carbohydrate reserves, e.g. in many legumes the net uptake of CO2 by the green pod is low due to the high rate of pod and seed respiration, at high temperatures net uptake can become negative. As plants acclimate to high temperatures the rate of respiration falls lessening the impact on net photosynthesis.
Imbalances can also occur due to the effect of temperature on physical processes, e.g. as temperature rises, the solubility of oxygen increases more than that of carbon dioxide, so oxygen becomes more concentrated in the cell solution compared to carbon dioxide. This imbalance contributes to the increase in the oxygenation of RuBP at high temperatures (i.e. an increased rate of photorespiration).
The high temperature sensitivity of reproductive development can be viewed as an imbalance. Detailed studies have found that the yield of certain cowpea varieties was limited at high temperatures due to reduced seed set. This limitation was mitigated by increasing sink demand through breeding with more heat tolerant varieties. The fact that seed set can be limited by the demand for assimilates at high temperature shows that the thermal sensitivity of the reproductive sink can be out of balance with than that of the photosynthetic source (http://www.plantstress.com/Articles/heat_m/heat_m.htm). A similar sink restriction is found in cereals grown at high temperatures, where grain development is restricted by its ability to convert the available assimilates into starch (Stone and Nicolas 1994).
14.7.4 - Heat tolerance
Where overheating cannot be avoided, plants have developed a range of mechanisms to tolerate high temperatures and to resist the stresses outlined above. Many of these mechanisms have been harnessed by plant breeders to develop more heat resistant crops (for review see: http://www.plantstress.com/Articles/heat_m/heat_m.htm). Each species has a different capacity to respond to heat stress. The degree of heat tolerance tends to follow the species’ native thermal range, with plants irreversibly damaged by temperatures between 30-40 oC termed ‘heat sensitive’ and those only damaged above 40 oC designated as ‘heat resistant’. A key aspect of tolerance to heat stress is the ability to acclimate and the mechanisms described below are typically up-regulated when plants are exposed to moderate heat stress (Figure 14.28).
Fig14.28.png
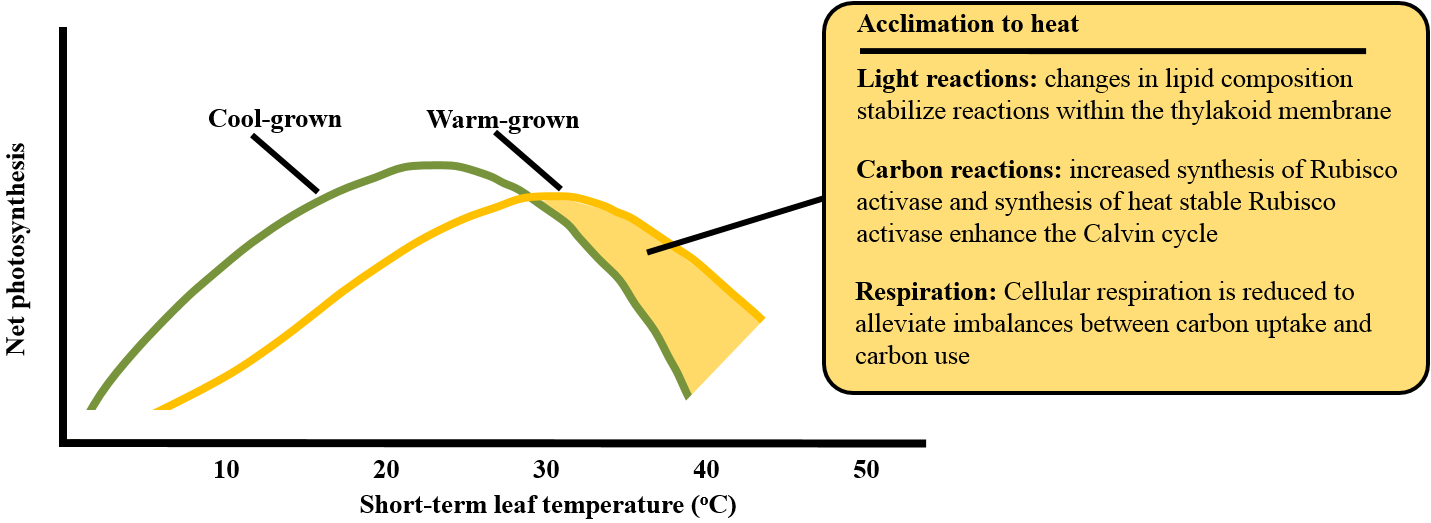
Figure 14.28. In heat tolerant plants, growth at warm temperatures results in acclimation of photosynthesis. Adjustments in membrane composition, protein synthesis, and metabolic regulation alleviate some of the effects of high temperature. Acclimation is mediated, in part, by an increase in expression of heat shock proteins. Based on Sage and Kubien (2007) and Yamori et al. (2014).
(a) Membrane state, structure and composition
In order to tolerate high temperatures, plants must maintain membrane fluidity within a biologically functional range (membrane thermostability). The degree to which membrane fluidity increases with temperature is dependent on membrane composition. Lipids that have unsaturated fatty acid chains, short fatty acid chains or a low sterol content generally form membranes that are more fluid and less stable at high temperatures. The sensitivity of membranes to heat stress can be reduced by increasing the proportion of saturated lipids or by altering the composition of specific lipids. Early work by Jim Lyons and John Raison at the CSIRO Division of Food Research highlighted the fact that tropical species tend to have a higher proportion of saturated lipids than temperate species, but found that the full role of lipid composition in regulating membrane fluidity was complex. Changes in lipid composition during acclimation to high temperature, including increases in the proportion of saturated lipids, have been described in cyanobacteria (Los and Murata 2004) and a number of plants from both warm and cool regions (Raison et al. 1982; Larkindale and Huang 2004). Some of the changes in the physical properties of membranes are regulated by the activity of heat shock proteins (see below), but others are not (Sharkey 2005).
Alteration of lipid composition through gene manipulation has been shown to increase heat tolerance in Arabidopsis, soybean and tobacco (Alfonso et al. 2001; Murakami et al. 2000). Murakami et al. (2000) produced transgenic tobacco plants with a reduced proportion of trienoic fatty acids (unsaturated lipids with three cis double bonds) in the chloroplast membranes. Exposure of the plants to 45oC for 5 minutes reduced photosynthesis by 50 % in the wildtype while the transgenic plants were unaffected (all plants showed complete inhibition of photosynthesis after exposure to 50oC for 5 minutes).
(b) Heat shock proteins
Within minutes of temperature rising above the optimum, the expression of most genes used for general metabolism is inhibited, however, a sub-set of specialised stress response genes are actively up-regulated. The best characterised of these genes are a multi-family group known as heat shock proteins (HSP). HSP occur in all organisms. In plants, they show differential expression in many tissues and many cell compartments. HSP utilise a novel transcription factor to respond directly to heat, and their levels have been shown to rise along with temperature until the lethal threshold temperature is reached. On exposure to high temperature HSP expression typically peaks after 1-2 hours and diminishes after 6-8 hours, after which the cell environment is modified enough for the transcription and translation of other genes to resume (Larkindale et al. 2005; Allakhverdiev et al. 2008).
Many HSP are thought to act as chaperone proteins, protecting other proteins from denaturation by reducing misfolding, unfolding, and aggregation. Chaperone activity also helps maintain the translocation of proteins across cell membranes. The up-regulation of HSP has been shown to improve tolerance to and recover from heat stress in several systems, e.g. addition of purified HSP of isolated tomato chloroplasts significantly improved their heat tolerance by protecting PSII electron transport (Allakhverdiev et al. 2008). This chaperone role can offer protection from stresses aside from heat, and HSP have been shown to be up-regulated by a variety of stresses that escalate protein denaturation. Larkindale et al. (2005), describe five classes of HSP the names indicating the molecular weight:
- HSP60 and HSP70 have been shown to act as chaperone proteins in plants and other organisms, and some also function to stabilise membranes preventing the loss of permeability.
- HSP90 are less well characterised in plants, they are thought to interact with signal transduction proteins that form part to the overall heat stress response.
- HSP100 act as chaperone proteins in conjunction with HSP60 and HSP70 and may perform other roles. Plants lacking HSP 100 can grow normal at optimum temperatures but are unable to acclimate during heat stress.
- Small HSP are particularly important in plants but are less well characterised than HSP60 and HSP70. Small HSP are a diverse group including several gene families that are targeted to different cellular compartments, including the cytosol, chloroplast and mitochondria. The function of many of the Small HSP is still unknown. Some may be involved in chaperone protein activity and some are involved in maintaining membrane stability including the protection of membranes essential for the functioning of PSII.
Certain HSP also act to clean up the cell, removing denatured proteins by increasing the proteolysis activity of ubiquitin. The removal of potentially toxic protein aggregations is thought to be a key part of acclimation to heat stress. Indeed, heat stress also stimulates the up-regulation of ubiquitin itself (Larkindale et al. 2005).
(c) Reactive Oxygen Species (ROS)
The impairment of metabolic function during heat stress results in increased production of ROS, which in turn causes secondary damage to proteins and membranes. Accumulation of ROS during heat stress has been associated with both the light reactions and the Calvin cycle reactions. The reaction centre of PSII is particularly vulnerable, producing superoxide radicals, hydroxyl radicals and hydrogen peroxide under heat stress. Antioxidant enzymes and non-enzyme systems serve to limit the formation of the most damaging ROS, such as singlet oxygen, and to detoxify the cells through ROS-scavenging. Although some antioxidant systems are impaired at high temperatures, others are up-regulated and can be considered part of the heat stress response (Sharkey 2005; Larkindale et al. 2005; Allakhverdiev et al. 2008).
(d) Heat stress response: other mechanisms
Although HSP form a critical part of the heat stress response, they still account for a small minority of the transcripts that are up-regulated during heat acclimation. Several groups are currently working to elucidate the role of other changes in the transcriptome, proteome, metabolome and lipidome in regulating signal transduction and response to heat stress (for review see Mittler et al. 2012). Among the metabolites associated with heat acclimation perhaps the best characterised are the compatible solutes (which also play a crucial role during water stress and salinity stress (see Chapter 17). In the case of heat stress, their primary role is thought to be similar to that of the chaperone proteins, i.e. the protection of protein and membrane function (Larkindale et al. 2005; Allakhverdiev et al. 2008). There is also increasing interest in the role of isoprene in heat tolerance. Isoprene is a small hydrocarbon sometimes released from plants in large quantities. It is particularly associated with certain tree species, such as eucalyptus. Indeed, it is isoprene that is responsible for the distinctive blue haze that characterises Australia’s ‘blue mountains’. Although the full purpose of isoprene has not been established, there is good evidence that it is involved in the development of heat tolerance. In particular, the production of isoprene has been shown to reduce the inhibition of photosynthesis during moderate heat stress, perhaps by associating with the thylakoid membrane to increase hydrophobic interactions and protect membrane function (Sharkey 2005).
14.7.5 References
References:
Alfonso M, Yruela I, Almarcegui S et al. (2001) Unusual tolerance to high temperatures in a new herbicide-resistant D1 mutant from Glycine max (L.) Merr. cell cultures deficient in fatty acid desaturation. Planta 212: 573-582
Allakhverdiev SI, Kreslavski VD, Klimov VV et al. (2008) Heat stress: An overview of molecular responses in photosynthesis. Photosyn Res 98: 541-550
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31: 491-543
Bewley JD, Black M, Halmer P (2006) The encyclopedia of seeds: Science, technology and uses: CABI Publishing.
Farrell AD, Gilliland TJ (2011) Yield and quality of forage maize grown under marginal climatic conditions in Northern Ireland. Grass Forage Sci 66: 214-223
John-Bejai C, Farrell AD, Cooper FM, Oatham M (2013) Contrasting physiological responses to excess heat and irradiance in two tropical savanna sedges. AoB PLANTS 5: doi:10.1093/aobpla/plt051
Larcher W (2003) Physiological Plant Ecology: Ecophysiology and stress physiology of functional groups. Springer Verlag
Larkindale J, Mishkind M, Vierling E (2005) Plant responses to high temperature. In: Plant Abiotic Stress. Ed MA Jenks, PM Hasegawa, pp. 100-144. DOI:10.1002/9780470988503.ch5
Long SP, Ort DR (2010) More than taking the heat: Crops and global change. Curr Opin Plant Biol 13: 240-247
Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37: 118-125
Murakami Y, Tsuyama M, Kobayashi Y et al. (2000) Trienoic fatty acids and plant tolerance of high temperature. Science 287: 476-479
Nobel PS (1988).Principles underlying the prediction of temperature in plants, with special reference to desert succulents. In: Plants and Temperature, 42, 1-23. Ed, SP Long, FI Woodward. Symposia Soc Exp Biol
Porter JR, Gawith M (1999) Temperatures and the growth and development of wheat: A review. Eur J Agron 10: 23-36
Raison JK, Roberts JKM, Berry JA (1982) Correlations between the thermal stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of Nerium oleander to growth temperature. Biochim Biophys Acta (Biomem) 688: 218-228
Sage, RF, Kubien SD (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Env 30: 1086-1106
Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid eactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28: 269-277
Stone PJ, Nicolas ME (1994) Wheat cultivars vary widely in their responses of grain yield and quality to short periods of post-anthesis heat stress. Aust J Plant Physiol 21:887-900
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosyn Res 119: 101-117 doi: 10.1007/s11120-013-9874-6
Further reading
Hall AE. Heat stress and its impact. In: Plant Stress. Ed A Blum. http://www.plantstress.com/Articles/heat_i/heat_i.htm
Hall AE. The mitigation of heat stress. In: Plant Stress. Ed A Blum. http://www.plantstress.com/Articles/heat_m/heat_m.htm