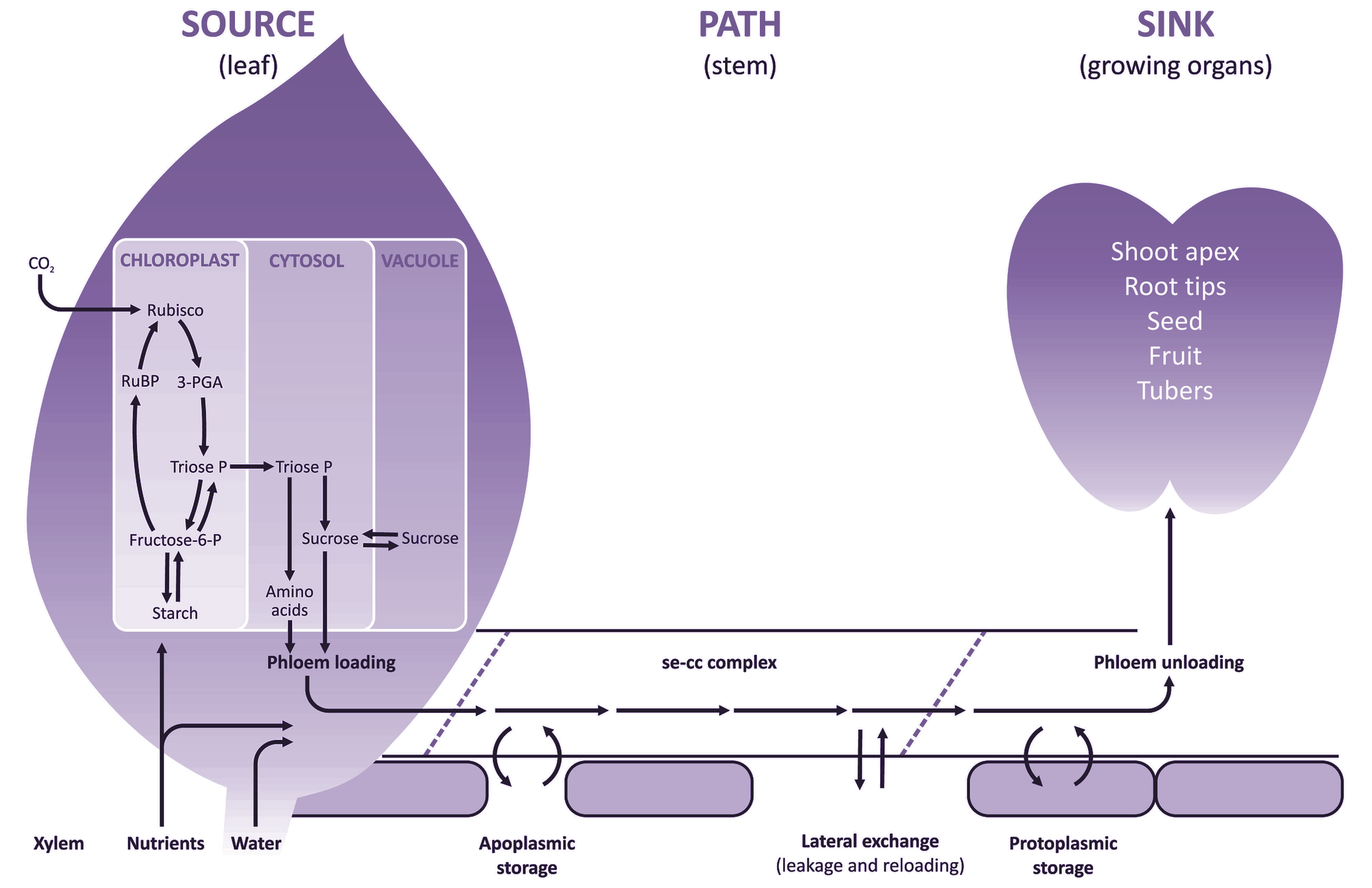
(a) Source processes
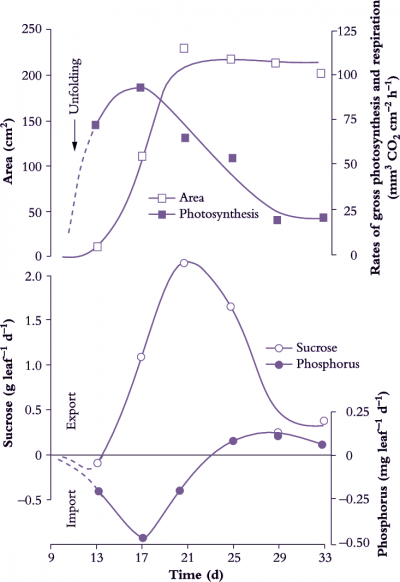
Net export of photoassimilates occurs from fully expanded leaves (Figure 5.2) and long-term storage pools located along the axial transport pathway. Chloroplasts of C3 plants (Chapters 1 and 2) partition photoassimilates between the photosynthetic oxidative cycle and starch biosynthesis or release them immediately to the cytosol as triose phosphate for sucrose synthesis. In non-starch-forming leaves, high concentrations of sugars can be accumulated in the vacuoles of mesophyll cells or made available for immediate loading into the phloem and export. Leaves also serve as secondary sources for nutrients and amino acids previously delivered in the transpiration stream. Nutrients and amino acids can be exported in the phloem immediately, or after accumulation in short-term storage pools.
An additional source of photoassimilates is located along the axial phloem path (petioles, stems, peduncles, pedicels and roots) as a result of leakage from the vascular tissues. Leaked photoassimilates accumulate in short- or long-term storage pools which serve as secondary sources to buffer photo-assimilate supplies to the sinks against shifts in export rates from the primary photoassimilate sources.
(b) Path processes
Assimilates including sucrose, amino acids are transferred into sieve elements of fully expanded leaves against significant concentration and electrochemical gradients. This process is referred to as phloem loading. The cellular pathways of phloem loading, and hence transport mechanisms and controls, vary between plant species. Longitudinal transport of assimilates through sieve elements is achieved by mass flow and is termed phloem translocation. Mass flow is driven by a pressure gradient generated osmotically at either end of the phloem pathway, with a high concentration of solutes at the source end and a lower concentration at the sink end. At the sink, assimilates exit the sieve elements and move into recipient sink cells where they are used for growth or storage. Movement from sieve elements to recipient sink cells is called phloem unloading. The cellular pathway of phloem unloading, and hence transport mechanisms and controls, vary depending upon sink function.
(c) Sink processes
Many sink organs are characterised by low rates of transpiration (an exception is a developing leaf) so that most assimilates are delivered by the phloem. Having reached the sink cell cytoplasm through the post-sieve-element transport pathway, assimilates are either metabolised to satisfy the energy, maintenance and growth requirements of sink cells or are compartmented into polymer or vacuolar storage. Collectively, metabolism and compartmentation create a demand for assimilates which is ultimately responsible for driving phloem import.