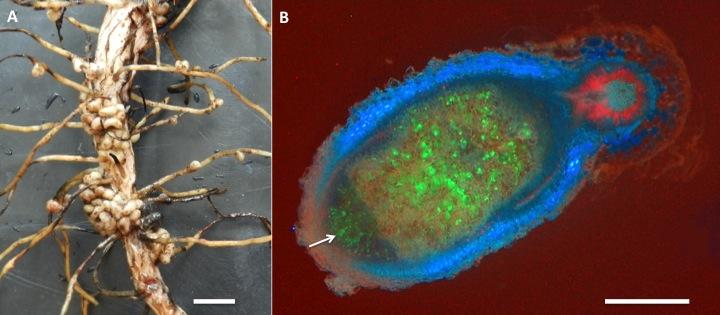
The symbiosis of legumes with rhizobia is the most effective and agriculturally the most important nitrogen-fixing symbiosis. Rhizobia are soil bacteria from the α- and β-proteobacteria (including Burkholderia sp.) that can occur free-living in the soil, but benefit greatly from symbiosis with legume partners, which provide a carbon source, shelter inside a nodule as well as a niche outside the species-rich and competitive zone of the rhizosphere (See previous section on the Soil-root interface). Many species of rhizobia only effectively fix nitrogen inside a nodule (Figure 4.41).
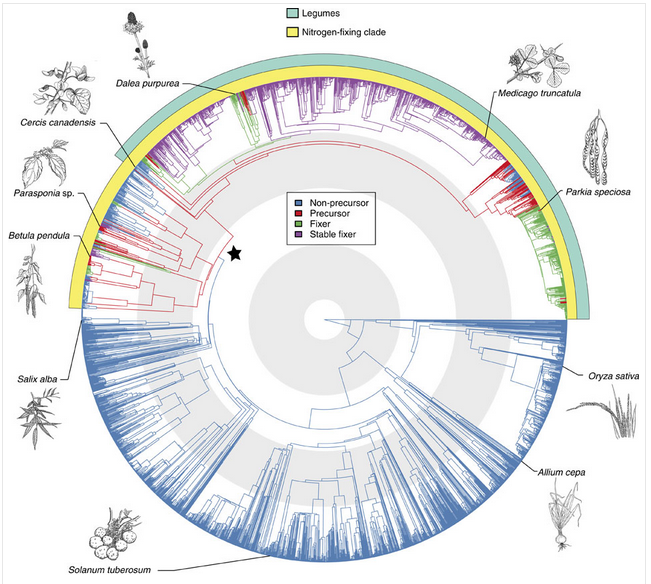
The ‘precursor’ of symbiosis evolved approximately 100 million years ago, and led to the similar symbioses of actinorhizal plants with Frankia, and the legume symbiosis with rhizobia. Nitrogen fixing symbioses in legumes evolved at a time of relatively high atmospheric CO2. Nitrogen fixation is likely to have conferred an advantage in using this increased CO2 for photosynthesis (Sprent 2007). Phylogenetic reconstruction of symbiotic nitrogen fixation suggests that it evolved once, and was subsequently gained and lost several times in legumes (Figure 4.42; Werner et al. 2014).
Nodules formed by members of the family Leguminoseae have a central zone of infected cells, surrounded by a cortex of uninfected cells. A root vascular strand branches within the cortex of the nodule. This structure is quite distinct from nodules of the cycads or actinorhizal plants, which have a central vascular bundle and an infected cortex (a typical root vascular anatomy). Different legume species display various nodule growth patterns, but they can be roughly classified as either of indeterminate growth (i.e. with an apical meristem and consequent elongated shape) or determinate growth (i.e. a spherical meristem which ceases activity at nodule maturity).
The association between rhizobia and legumes is a controlled infection. Typically, the bacterial partner infects the plant through root hairs, and is then encapsulated by polysaccharide material produced by the host plant, forming infection threads. Infection threads then grow into the root cortex, while bacteria multiply within each thread. Finally, bacteria are released from the infection threads and engulfed by plant cells in a form of phagocytosis. This process results in a bacterium (sometimes several) encapsulated by a plant cell membrane. Encapsulating membranes control the delivery of photoassimilate to bacteria, thus ensuring a symbiotic rather than a parasitic relationship. These units are termed ‘symbiosomes’ (see also later Section 4.4.5)
Evolution of this partnership might be similar to that of other endosymbiotic organelles such as mitochondria and chloroplasts. Perhaps a future step in the evolution of a legume–rhizobium symbiosis will be retention of bacteria within plant cells to create a new organelle! If this were to happen, the legume would no longer be dependent on the presence of a microsymbiont for infection. Cells could maintain a low resident population of the new organelle, like plastids in non-photosynthetic tissue, and allow proliferation under set conditions within nodule structures.
In some legume symbioses, bacteria are not released from infection threads. This character is one of several that distinguish each of the three legume subfamilies Caesalpinoideae, Mimosoideae and Papilionoideae (e.g. cassia, acacia and soybean, respectively). The Caesalpinoideae are largely trees or shrubs, and the few species which nodulate have little nodule mass proportional to plant biomass (Sprent and Raven 1985). In most of the caesalpinoid species that do nodulate, the microsymbiont remains encapsulated in an infection thread throughout the life of a nodule. In some species the infection threads are thin walled, while in others bacteria are released into the cytoplasm. The Papilionoideae is considered the most advanced of the legume subfamilies.
Biological interactions between host plant and bacterium are subtle. Just as legumes vary genetically, so do the rod-shaped bacteria (rhizobia) that infect various legumes. Not all rhizobia are equally infective (able to infect and form nodules) or effective (able to fix N2) on all legumes. An appropriate bacterial partner must therefore be matched genetically with each legume for optimal N2 fixation. Pure cultures of rhizobia are produced commercially, generally in a peat-moss-based medium or as a seed coating, for inoculating legume seed prior to planting.