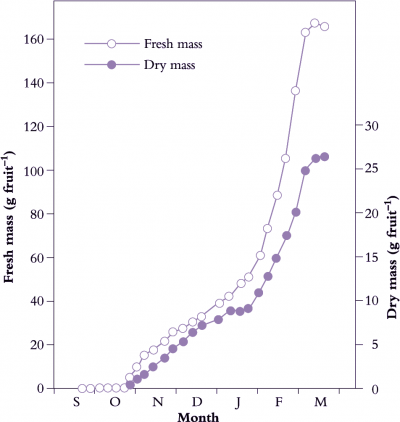
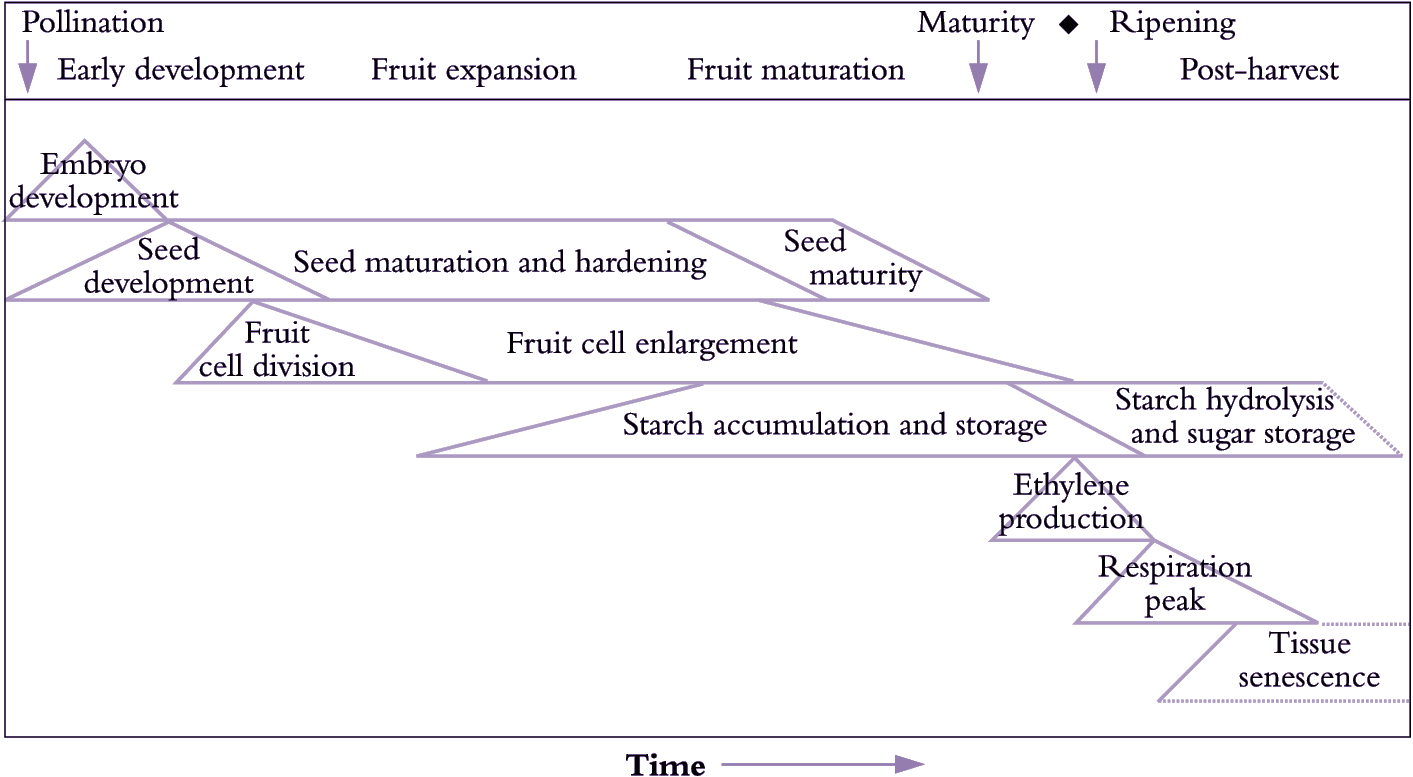
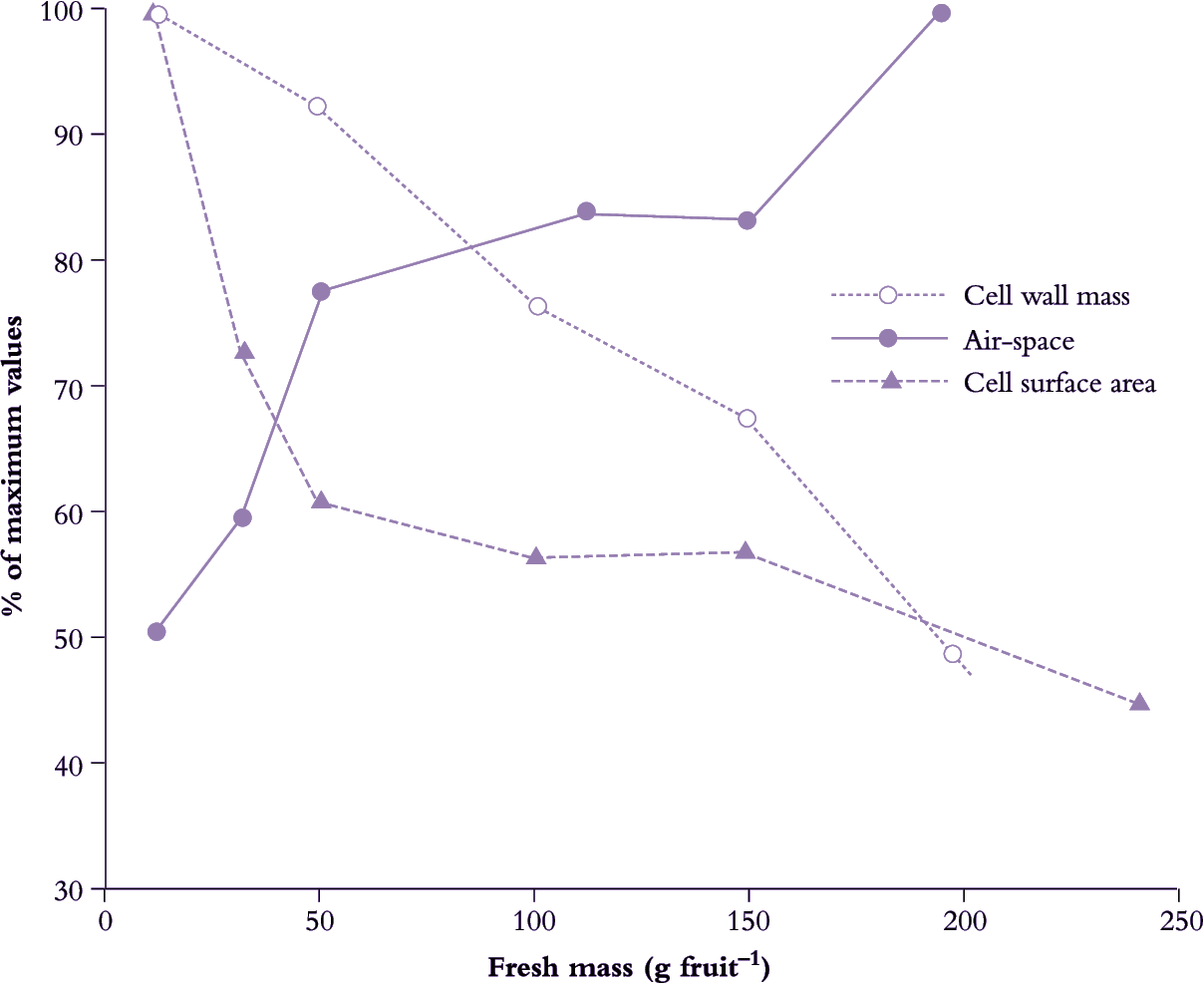
Fertilisation is generally crucial for fruit set and pericarp development (Figure 11.1). As fertilised ovules develop into seeds, this influence on pericarp growth continues where production of hormones by the endosperm and developing embryo promotes pericarp growth. Indeed, there is usually a positive correlation between the number of seeds in the fruit and final fruit size (de Jong et al. 2009). The importance of seeds as sources of hormones for initiation and stimulation of fruit growth is implied by fruit response to exogenous hormones in parthenocarpic systems (development of fruit without seeds).
Applying auxin and gibberellins to unfertilised embryos is one way of achieving parthenocarpy; another is to use auxin transport inhibitors such as chloroflurenol to prevent loss of auxin from embryos so that a threshold level for pericarp response is exceeded. Studies of parthenocarpy in tomato and cucumber indicate that high auxin levels enhance embryo cell division, and this cell division phase seems to be more critical than subsequent cell expansion in determining final fruit size.
Such results imply a cooperative mode of action where gibberellins combine with auxins to initiate cell division. Seed cytokinins and cell division are similarly related because tomato seeds accumulate cytokinins that subsequently influence cell division in surrounding pericarp tissue (Gillaspy et al. 1993).
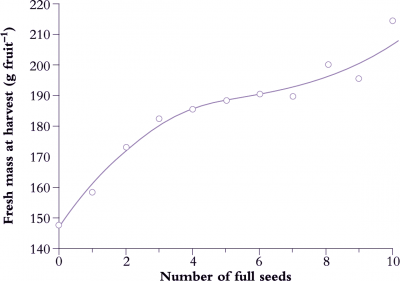
Such interdependence between seed development and fruit growth shows up in final fruit size. Parthenocarpic fruit have reduced auxin content and are generally smaller than wild-type fruit. In apple fruit, seed numbers frequently correlate with fruit growth (Figure 11.6) or with shape and size of fruit. Inadequate pollination of kiwifruit (Figure 11.1) results in distortion, and a curvilinear relationship emerges between seed number and fruit weight. A similar response is obtained when young seeds are surgically removed from immature strawberry fruit, causing a corresponding distortion in flesh development.
Despite ample evidence that natural control of fruit shape is primarily exerted by plant hormones originating from seeds and stimulating growth to varying degrees, this is not true for all fruit. In banana, fertile seeds actually suppress development of the fleshy pulp. In this anomalous case, fertilisation failure allows an ovary to grow.
In marrow, tomato and kiwifruit, ovary shape dictates spatial distribution of seeds. They in turn influence pericarp growth, so that fruit size and shape then become a function of initial ovary shape plus subsequent fertilisation and seed development.