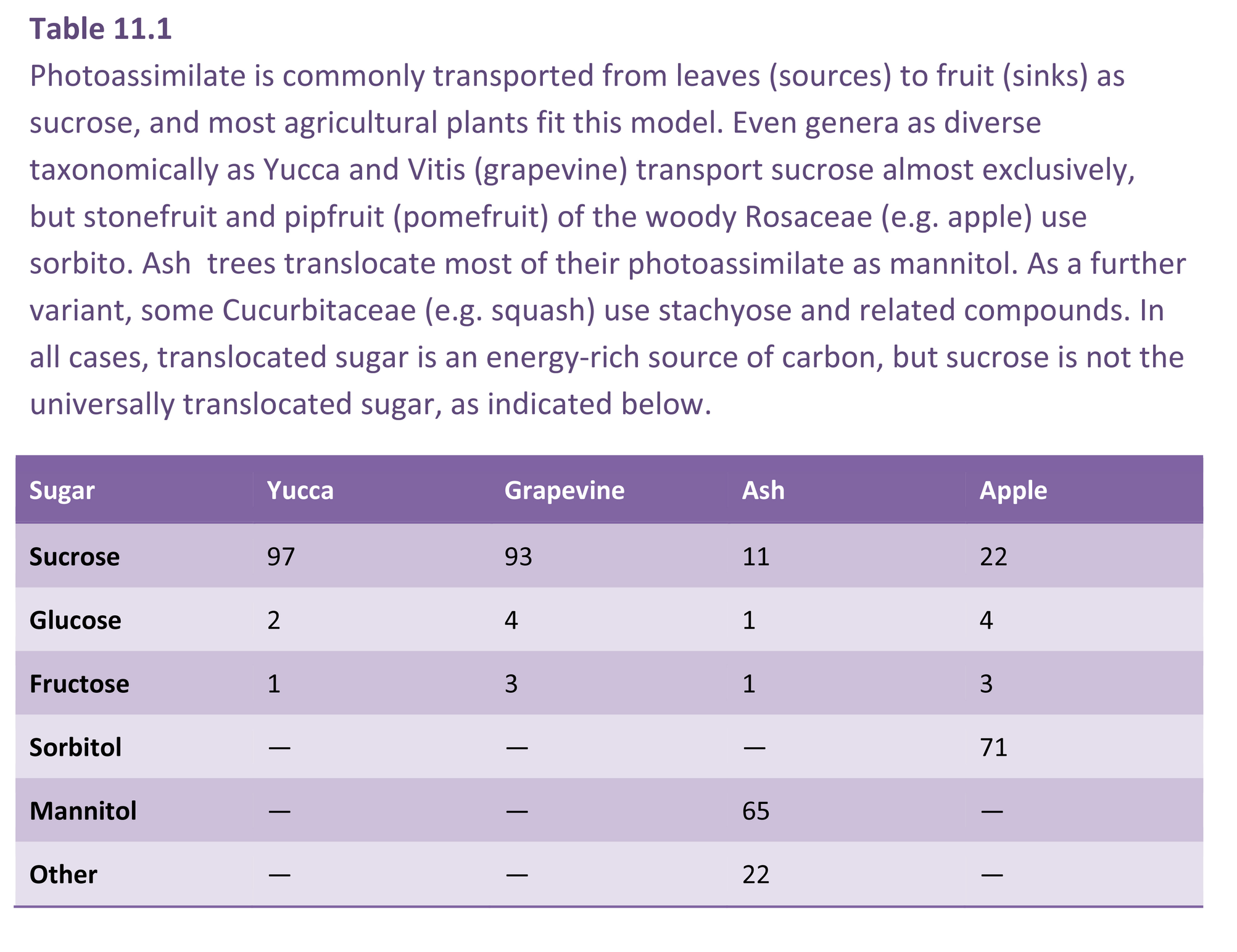
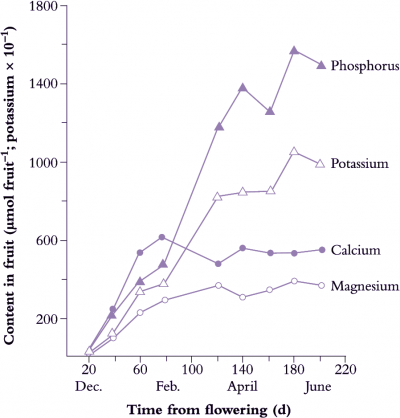
Carbon transport and subsequent metabolism in developing fruit cannot be viewed in isolation, particularly when aspects of fruit quality, such as taste and flavour, are directly dependent on such processes. In particular, sugar–acid balance and contents are primary determinants of the taste attributes of fruit, and so are of major significance for consumers. Too much acid and the fruit is tart and unpalatable; too little and the fruit is insipid and bland. In horticultural terms, acid levels are often expressed as titratable acidity (TA), and this is used as one indicator of taste. Another indicator used is the refractive index of the expressed sap (recorded as °Brix). This is a measure of the soluble solids concentration (SSC %) of expressed juice and represents the sum of organic acid, salts and sugar contents. Several organic acids may be present, but certain ones are characteristic of particular species or cultivars. For example, malic acid predominates in pipfruit (pomefruit), citric acid is dominant in citrus, while tartaric acid is dominant in grape. In kiwifruit, malic, citric and quinic acids are the major ones, and in total may exceed 1.5% of the fresh weight.
Acids are not transported into fruit via phloem connections, but are synthesised in situ. Part of the acid component comes from metabolism of the sugar imported through the phloem, but part can be synthesised by local fixation. In citrus, dark fixation of CO2 by mature fruit makes a meagre contribution to acid balance, but inter-conversion of imported carbon is of more consequence. In that case, citrate synthase and subsequent enzymes in the citric acid cycle appear to determine whether imported carbon (as sucrose) is transformed into other sugars or is metabolised further to organic acids.
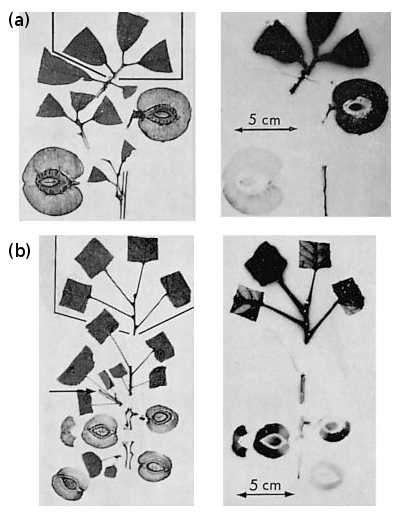
Starch–sugar balance is a major factor in consumer perceptions of fruit quality. In many fruit, including apple, banana and kiwifruit, starch accumulates throughout development, being laid down as granules in plastids. In kiwifruit, starch may reach 50% of the total dry matter towards the end of fruit growth (at about 15 weeks after pollination). As fruit approach maturity (17–20 weeks after pollination), there is a rapid onset of starch hydrolysis. Starch content at the onset of this conversion is not enough to account for all the sugar present in ripe fruit, and this implies that maturing fruit continue importing sugar up to harvest. Continuing import of 14C-labelled photoassimilate into maturing peach and apricot fruit confirms that pattern (Figure 11.7).
The dynamic between starch breakdown and soluble sugar increase can be a critical index of fruit maturity. ‘Hayward’ kiwifruit, for example, are judged to be mature enough to be harvested and to ripen properly if their soluble solids levels reach a specific target value of 6.2%. Starch pattern tests are used as maturity indices for some apple cultivars. For kiwifruit, the starch content of fruit at harvest may vary according to season, growing system, and cultivar. Starch content is strongly linked with sugar content of ripe fruit and hence with consumer perceptions of fruit taste. Starch content of fruit at harvest (commonly estimated from fruit dry matter content) is used commercially as a proxy for potential fruit taste.
As an additional factor in their dietary appeal, fruit are rich sources of vitamins, particularly vitamin C (L-ascorbic acid). Moreover, vitamin C can be a major metabolite (greater than 2 g kg–1 fresh weight) in fruit such as acerola, rosehip, quandong, kiwifruit, citrus, blackcurrant and guava, and has strong anti-oxidant properties. This may account for a notable absence of browning in kiwifruit and citrus when sliced (in conjunction with relatively low levels of polyphenols and polyphenol oxidase in those tissues). Vitamin C levels increase in the fruit during early growth, and tend to be stable through to maturity.
A number of other important vitamins have fruit or seeds as their major sources in the human diet. The B group vitamins such as B1, B2, pantothenic acid and biotin are present in both fruit and seeds, while B3 and B6 are particularly abundant in seeds. The vitamin A precursor β-carotene is found in useful quantities in some fruit, for example peach, apricot, melon and cherry.
Phenolics such as anthocyanins and tannins are also important in fruit and are responsible for much of the visual appeal of intact fruit (e.g. tamarillo), exposed flesh (e.g. cherry) or extracted juice (e.g. guava). They also contribute to flavour characteristics, adding a slight and pleasing astringency (as with the dessert apple) or a more aggressive one (as with cider apple and green banana).
Tannins in persimmon fruit are a special feature of that fruit and provide an interesting example of the potential dominance of a single quality characteristic in determining how a given fruit is used. The first cultivars of persimmon originating in China were markedly astringent, having high soluble tannin levels that made the fruit inedible until the tannins became condensed during the softening stages of ripening and early senescence. These original cultivars were therefore not eaten until the fruit flesh had become a glutinous paste. Later selection in Japan produced non-astringent cultivars such as ‘Fuyu’ that lose their astringency during the later stages of maturation, so that they can be eaten in a firm crisp state more typical of a fruit like apple. In persimmon, water-soluble tannins are compartmented in specific tannin cells of the mesocarp tissue. Tannin accumulation ceases with cell growth, and in non-astringent cultivars astringency declines both through soluble tannin dilution and through polymerisation, where soluble tannins are condensed into an insoluble form.