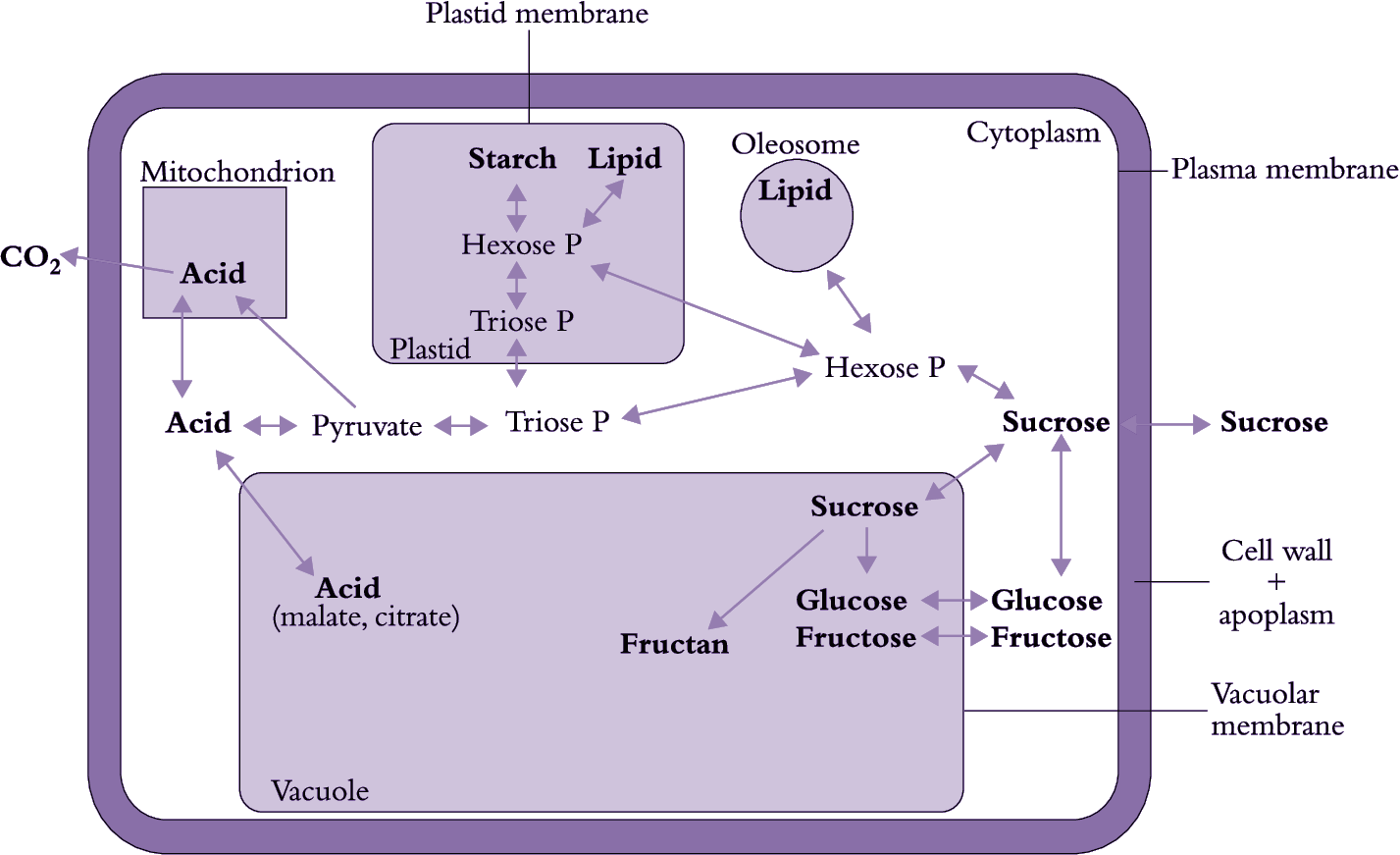
During development, photoassimilate is stored in fruit (and other sink organs such as root vegetables, seeds and flowers) in either a soluble or insoluble form (Figure 11.9). Fruit that store carbon in a soluble form (e.g. berry fruit, peach, persimmon, melon, grape, citrus) need to remain on the plant until nearly ripe if they are to survive postharvest storage and meet customer expectations. In most cases the major and rapid increase in soluble sugar content does not occur until late in development, signalling the beginning of ripening. Because the sugar source is the parent plant, harvesting such fruit too early reduces their final sugar content to unacceptable levels. In contrast, there are other fruit that store their carbon in insoluble forms, particularly starch. This allows greater efficiency in accumulating carbon, as the storage product is more compact, osmotically inactive and better segregated from metabolic processes. Examples are avocado, which stores carbon as both starch and lipid, and kiwifruit, apple, pear, mango, papaya and banana, all of which store carbon as starch.
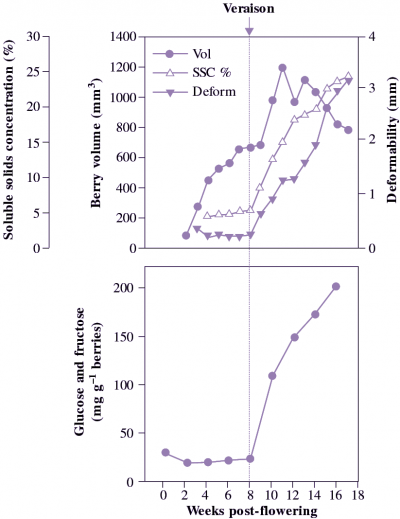
In fruit that store carbon as sugars and organic acids during development, colour changes followed by the initiation of softening signal that fruit are becoming mature and ready to harvest. The challenge facing postharvest physiologists is to assess when such fruit are still sufficiently firm to allow easy handling and storage, but have enough sugar for a true ripe flavour to develop. A developing crop is typically monitored with a simple refractometer test (soluble solids concentration based on refractive index of expressed juice). In grape, a rapid rise in sugar content (beginning at ‘veraison’) may need to reach SSC values of around 20% (with, say, 17% sugar and 2% acid as the main soluble components) before a successful harvest can be assured.
There is more to good flavour than high sugar content, with sugar to acid ratio being particularly important, so that a combination of SSC to acid ratio with a minimum SSC level may be required. For example, New Zealand mandarins may not be exported to Japan if the SSC to acid ratio is below 10 (when the proportion of acid is too high). In other crops such as melon, the acid component is unimportant, and sugar content primarily dictates fruit quality.
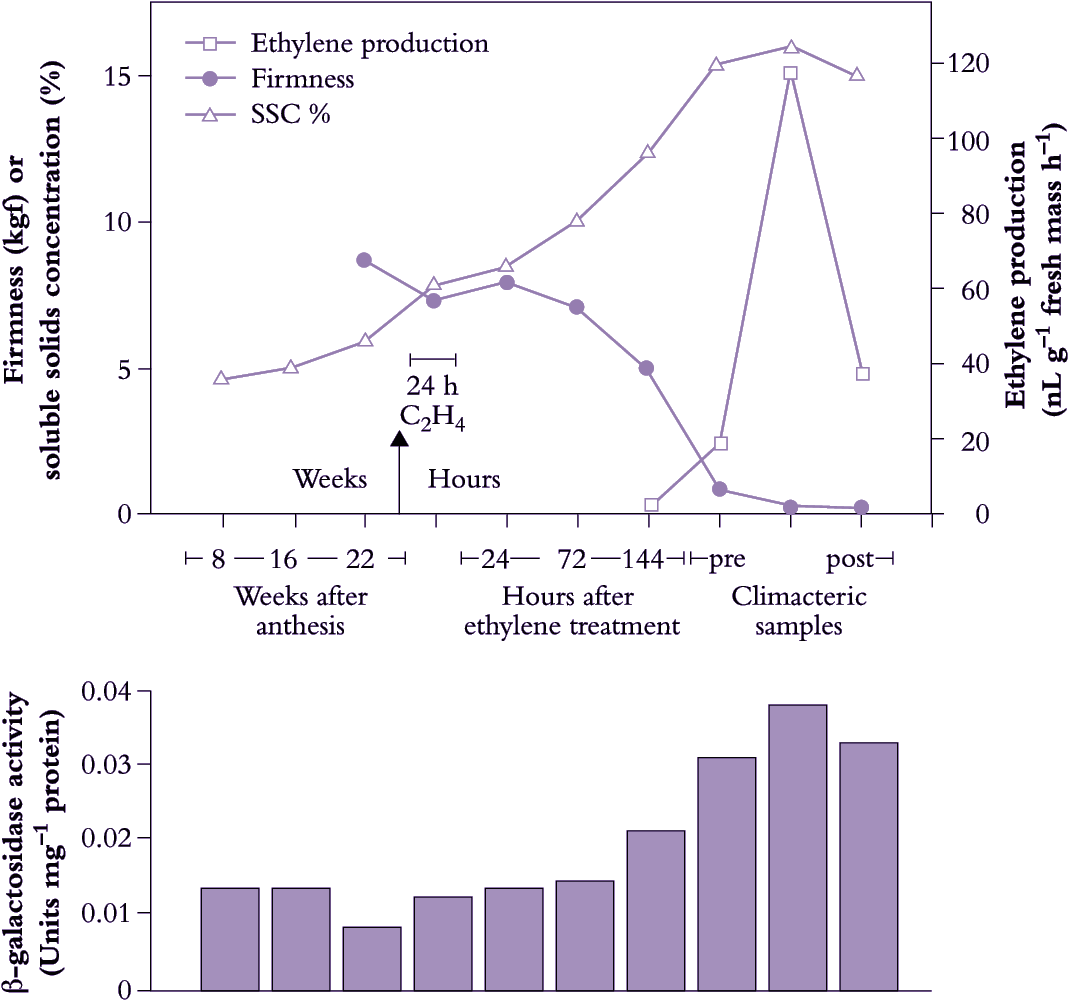
In fruit that store carbon as starch, time of picking is less dependent on sugar content, since a doubling or more of sugar concentration by starch hydrolysis can still take place after the fruit are picked. Once starch utilisation has started, fruit can be picked without much detriment to final eating quality. SSC measurements alone are then insufficient, and measurements of total solids, dry matter, oil content and starch concentration are used as well. For example, in kiwifruit sequential measurements of SSC are combined with dry matter measurements; in apple and pear, the pattern of starch distribution within the fruit is recorded along with SSC and ethylene measurements; in mango, total solids (and fruit shape, flesh colour and firmness) are recorded; in papaya, colour changes and sequential SSC measurements are recorded; in avocado, dry matter, sometimes in combination with oil content, is used. In banana, shape or ‘fullness’ of fruit is an important criterion rather than starch level because bananas can be harvested over a remarkably wide range of maturities and still ripen satisfactorily.
Thus, when fruit store carbon in insoluble forms, they can often be harvested while still hard, lending greater flexibility to postharvest handling and ensuring a longer storage life.