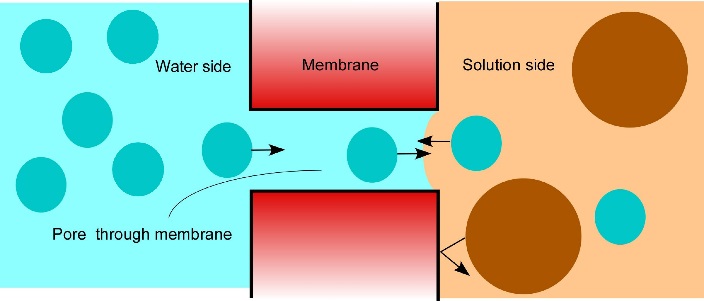
Figure 7.24 How osmosis works. Water is on the left shown as blue dots. An aqueous solution is on the right shown as a mix of blue dots and large brown dots (solute). A porous membrane separates the two compartments but the pores are too small or too selective for the solute to move through. Instead, the solute is reflected by the membrane. The water side contains more water than the solution side because the solute occupies space that otherwise would be occupied by water. Consequently, more water enters the pores from the water side than from the solution side. As long as there is a concentration difference on the two sides of the membrane, more water will cross from the diluter side than from the concentrated side. (Diagram courtesy JS Boyer)