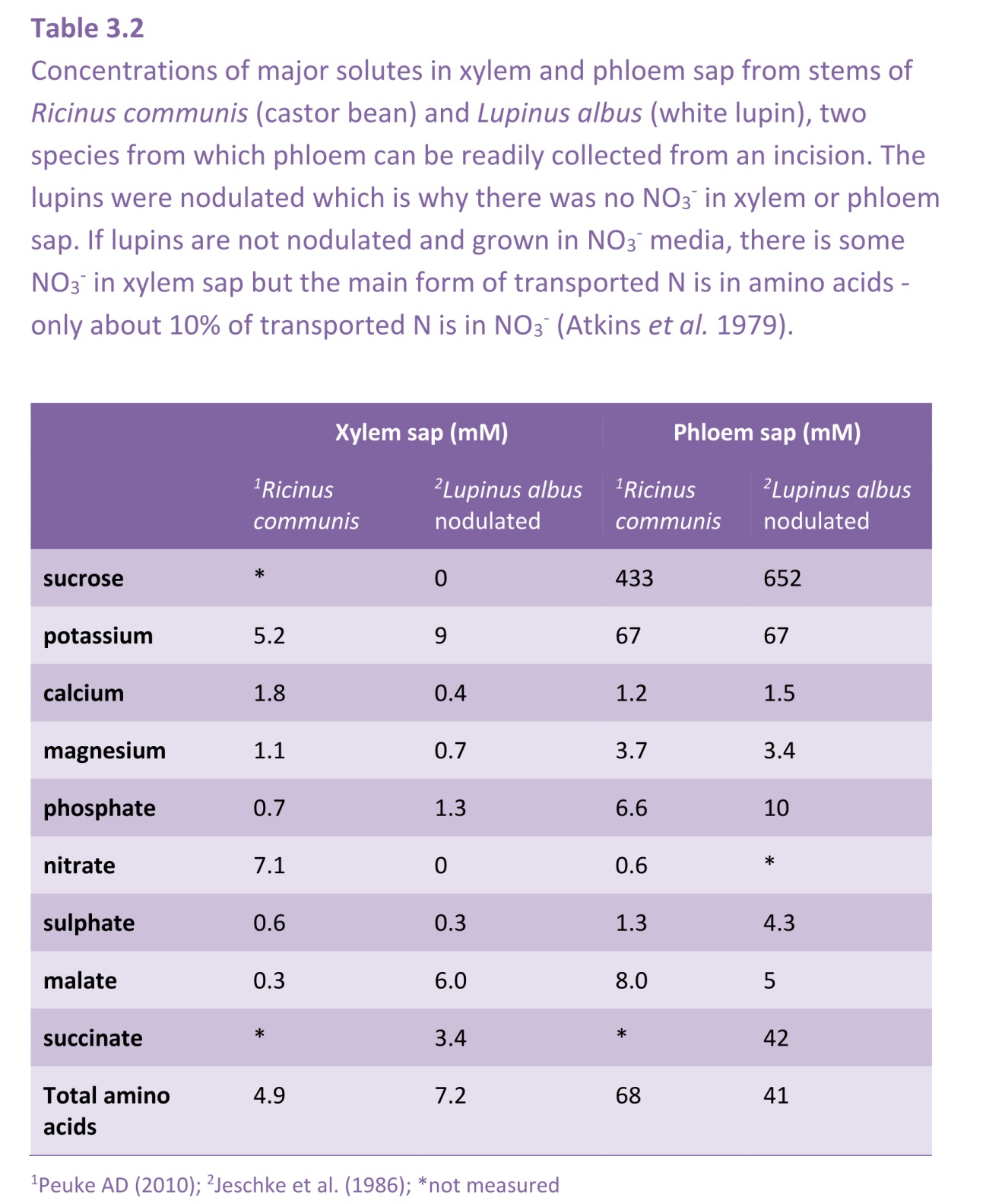
Around 1905, great plans were made to resolve the mystery of the ascent of sap in trees by Professor E.J. Ewart in Melbourne, using eucalypts as a model plant. At that time, Australian mountain ashes (Eucalyptus regnans) vied with American coast redwoods (Sequoia sempervirens) as the tallest trees in the world, being well over 100 m tall. Using special scaffolding, Ewart climbed eucalypt trees, removed lengths of branch and measured the pressures required to push water through these stems. These investigations led him to conclude ‘The ascent of water is, therefore, a vital problem in so far as it depends upon conditions which hitherto can only be maintained in living wood’. If water transport required living cells, it could not be supported by discovery of a pump akin to that in animals. Even roots, which sometimes could pump water by root pressure, lacked the necessary positive pressures to push water so far aloft, especially around midday when water was most needed.
Suction from the shoots was an alternative explanation, but manmade suction pumps cannot do this without inducing formation of air bubbles (embolisms) in the xylem and blocking flow. One clue to the solution came from Dixon and Joly (1894) who claimed that very pure water molecules would be held together by powerful cohesive forces provided the water was especially clean (much cleaner than in manmade pumps).
Ewart did not agree with the unorthodox proposal that the suction of pure water through xylem vessels underpinned transpiration. However, Dixon (1914) ultimately postulated the Cohesion Theory, based on those properties of water which distinguish it as an ideal biological solvent. Cohesion (due to hydrogen bonding between molecules of water), adhesion to walls of the vessels, and surface tension, are central features. In short, in the absence of microscopic gas bubbles water could withstand quite enormous tensions.
Evaporation from wet cell walls of substomatal cavities in leaves creates a large tension (also called negative pressure or suction), which is transmitted via xylem conduits, pulling more sap from roots to leaves. Fine pores in cell walls provide sufficient suction to draw water to the crown of even a lofty tree: a curved interface in a 10 nm pore can store a pressure of -30 MPa. This value can be derived from equation (5) in the previous section: DP = 0.15/r where P is in Pa and r in this case is 5 x 10-9 m.
Through the evaporative power of the atmosphere, a continuous ‘chain’ or ‘catena’ of water, well below atmospheric pressure, could be drawn up to a leaf canopy. The tensions created in this way could even suck water from the surrounding soil. We now recognise that the evaporative energy is supplied as the latent heat of vaporisation ultimately derived from solar radiation. This cohesive property of water gave rise to the ‘Cohesion Theory for the Ascent of Sap’.
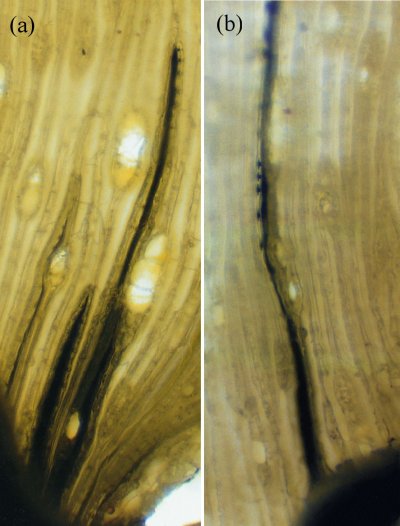
Two other properties of water are also essential for long-distance water transport: surface tension, and the adhesion of water to solid surfaces such as the xylem vessels within trees. Dixon claimed that if water could ‘hang together’, the enormous evaporative energy of the air (the same power which dries the washing hanging on a line) could be harnessed to lift sap, which is mainly water, vertically. This would entail no metabolic energy on the part of the plant. This theory of sap flow accorded with earlier experiments by Professor E. Strasburger in 1893 showing that a tall oak tree trunk, severed at the base, could draw poisons and dyes up to the leaves by some wick-like action. If metabolism energised sap flow, poison should have inhibited it. This was well illustrated in later experiments (Figure 3.11a) in which mercury was drawn through fine tracheids of pine stems purely through the suction created by transpirational water loss from the shoot above. The tension required to achieve this is about 2 MPa.
However, the physical properties of plants had to be more complex than those of simple pipes conducting water. As mentioned, manmade pumps failed through embolisms if used to suck water higher than 10 m, whereas hundreds of litres of water reaches the canopies of tall trees daily. Even overlapping sawcuts in tree trunks, which should allow a massive quantity of air to flow into xylem vessels when under suction and cause trees to die from embolisms, did not stop all water flow to leaves. If water was under such suction, how could trees keep air bubbles out of the sap when the trunk was cut? This additional problem was not resolved until the very complex anatomical structures of trunks were much better understood. The highly compartmentalized, extensively redundant structure of the xylem network performs the critical role of isolating gas voids while water transport continues in adjacent conduits. In reality, the complex structure of the xylem network is what makes reliable water transport under tension possible.
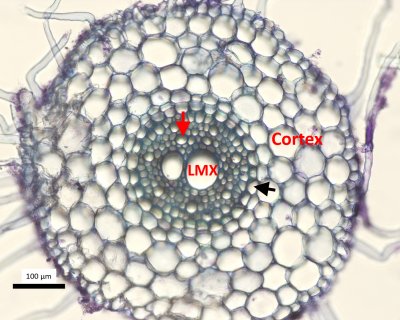
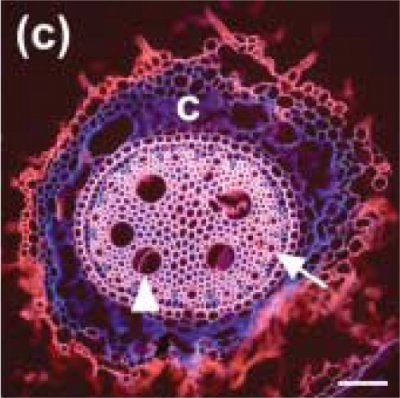
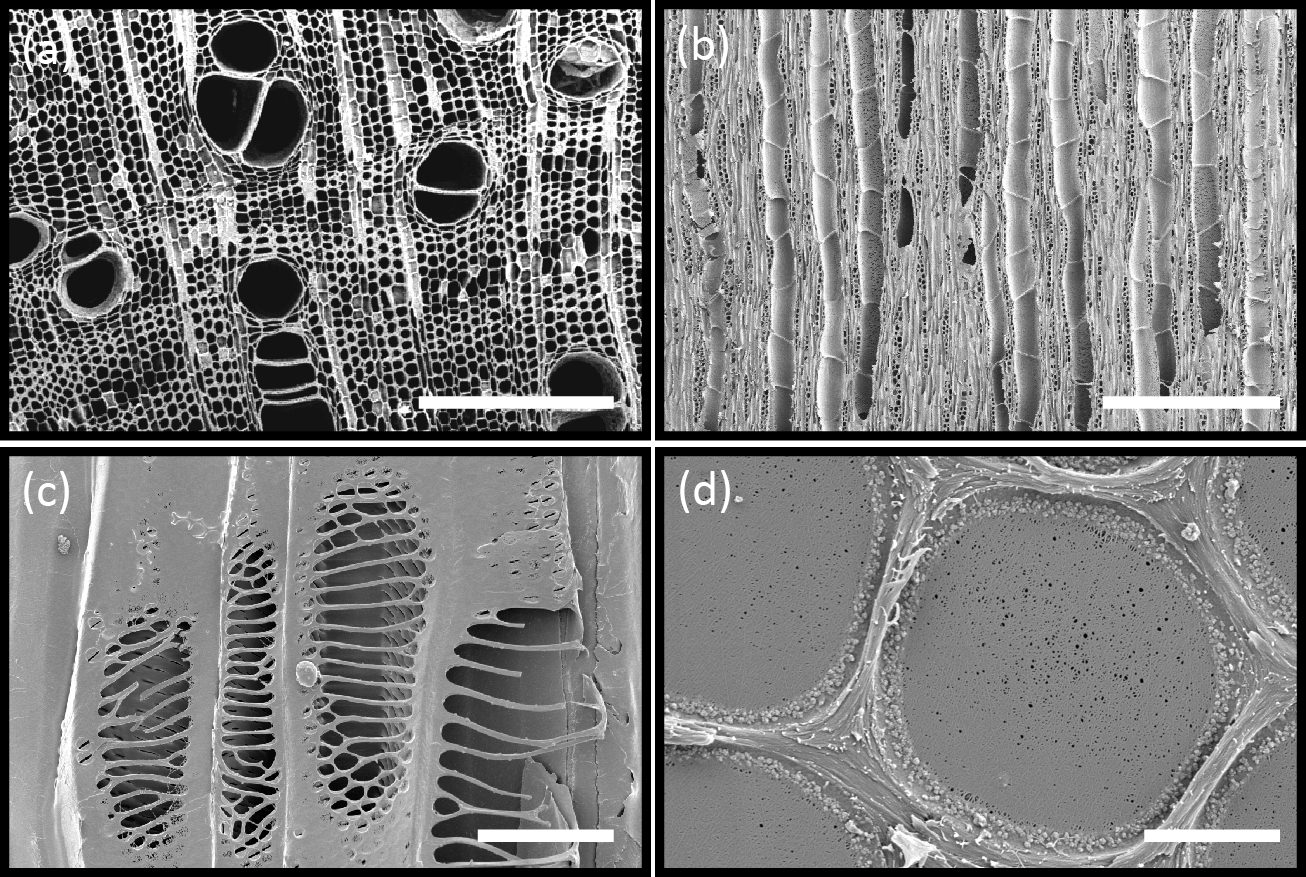
Xylem is not composed merely of pipes: it is made up of partially sealed units (technically vessels, tracheids and fibres, called collectively conduits), which most effectively limit the spread of introduced gases and thus, maintain water flow in some conduits despite very severe disruption from embolisms in others.