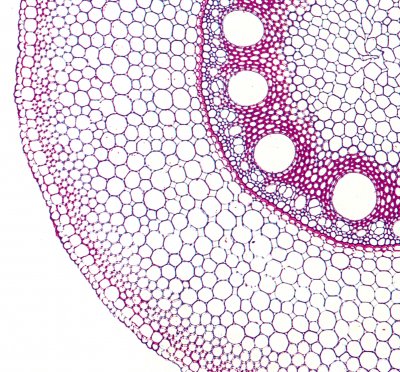
The epidermis and root hairs probably mediate most of the selective uptake of solutes from the soil solution. Nutrients are selectively taken up, and potential toxins excluded. The cortical cell layers continue this selective uptake if solutes travel apoplastically towards the stele.
The endodermis is the innermost cortical layer that surrounds the central vasculature, and forms a barrier preventing water flow and free diffusion of solutes in the apoplast because of its Casparian strip and suberin lamellae. It thus, prevents soil solution moving into the stele without crossing a membrane, and also prevents water from within the stele being lost back to the soil at night. Although, in some regions of the endodermis that are opposite xylem poles, water and solutes may bypass the endodermis through unsuberised “passage cells”. The endodermis also functions in structural support for the stele, particularly in drying soil, and minimises shrinkage or swelling of the cells of the stele. Its role in ion selectivity is minor compared to the cells of the cortex and epidermis.
In many plants, the cortical layer under the epidermis (the hypodermis) develops Casparian bands and suberin lamellae, and develops into the exodermis. This forms another barrier for the apoplastic movement of water and solutes. Just like in the endodermis, some passage cells in the exodermis may be free of suberin and can take up water and solutes.
(a) Endodermis
An endodermis with a Casparian strip is a feature of roots of all land plants from ferns upwards. As the innermost cortical layer that surrounds the central vasculature of roots, the endodermis acts as a barrier to the free diffusion of solutes from the soil into the stele, and the stele into the soil. The protective functions range from efficient water and nutrient transport to defence against soil-borne pathogens. The genes and regulation mechanisms that drive the differentiation of this intricately structured barrier have been reviewed by Geldner (2013).
The development of the endodermis has three stages: the primary stage in which the Casparian strip forms, the second stage when suberin lamellae encases the entire endodermal cell, and the tertiary stage when the inner tangential walls thicken and a layer of cellulose is deposited over the suberin lamellae.
The Casparian strip is made of lignin and suberin and deposited as a ring in the radial walls of the endodermal cells, like a hoop around a barrel of beer. It reaches from the plasma membrane to the outermost part of the wall and adjoins adjacent cells so there are no air spaces between the cells at this point. It therefore blocks the flow of water through the cell walls. It differentiates as root cells mature about 5-10 mm from the tip, and so an entirely apoplastic pathway from soil to central stele can occur only in very young root parts or at sites where the Casparian strip is disrupted such as site of lateral root development, which starts at the pericycle, the cell layer beneath the endodermis.
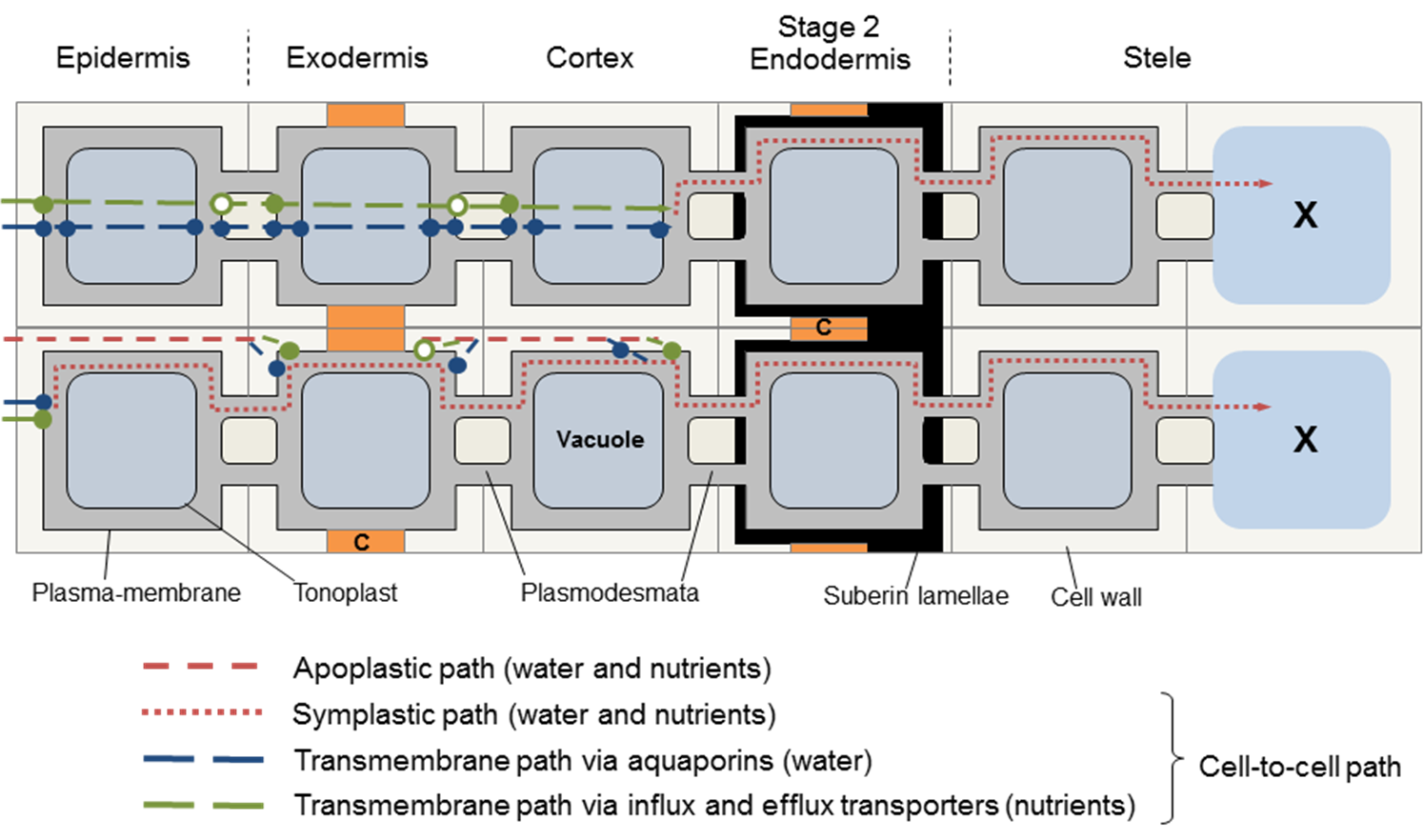
In the first stage of endodermis development, the Casparian strip in the primary wall prevents flow of water and solutes through the wall from inner cortex to stele. Any solutes remaining in the apoplastic water must enter the endodermis via membrane transporters. Water in the apoplast must enter the endodermis via aquaporins as illustrated in the lower pathway shown in Figure 3. 50.
In the second stage of development, the endodermis becomes suberised as the secondary walls develop, and suberin lamellae are formed all over the cell, underneath the primary wall that contains the Casparian strip. This occurs at varying distances from the root tip depending on species and is often induced under drought to form in younger parts of the root.
Suberin is a hydrophobic polymer, deposited in the secondary cell wall in lamellae. It therefore seals off the plasma membrane from solutes, as water and ion channels are sealed. So the transcellular and apoplastic pathways are curtailed, and water and solutes enter the endodermis only through plasmodesmata from neighbouring cells (Figure 3.51). Any function in selective control of particular nutrients into or out of the endodermal cell through the suberin lamellae is unclear.
Some endodermal cells remain at the primary stage even late in root development, and are called passage cells.
Stage 3 of the endodermis involves further deposition of cellulosic wall material, further impeding flow of solution through walls.
(b) Exodermis
Exodermis is the name given to a hypodermis with Casparian strips and suberised lamellae.
Occurrence
Roots of most species form an exodermis with time. First, a Casparian band is laid down in the primary wall of the hypodermis, then all the wall is suberised especially the inner wall, as for the endodermis. Some cells in this hypodermal layer (‘passage cells’) remain unsuberised.
The development of the exodermis is very common in the plant kingdom (Perumalla et al. 1990). In a study of 180 angiosperm species, the great majority (89 %) showed a clearly suberised exodermis with Casparian strip. It was found in roots of primitive and advanced plant families, from hydrophytic, mesophytic and xerophytic habitats, but was lacking in some Poaceae. It was notably absent in oat, barley and wheat (Perumalla et al. 1990), but generalizations within cereals cannot be made as subsequently maize was found to have an exodermis (Hose et al. 2001),
The Casparian band can develop close to the root tip. For example, in aeroponically grown maize a complete exodermal layer formed 30 mm above the root tip. In roots elongating more slowly due to abiotic stress or low temperature, it can be found closer to the tip. The extent at which apoplastic barriers form depends on the stage of development of the root system and also the habitat: drought, waterlogging, salinity, nutrient deficiency or toxicity may strongly influence the degree of suberisation (Hose et al. 2001).
Permeability to water and solutes
When an exodermis forms, it also imposes a restriction to radial transport. Complete layers of suberin constrain water and solute flow only via plasmodesmata from the epidermis and inner cortical cells. Whether or not it can be considered as a barrier depends on the degree of suberisation and the number of passage cells within it. Its properties as a barrier are variable (Hose et al. 2001).
The exodermis represents a resistance to the radial flow of both water and solutes, much like the endodermis in Stage 1 development. It restricts radial apoplasmic movement and may also restrict transmembrane transport of nutrients. Exodermal layers become functionally mature 20–120 mm from the apex, where lateral roots are initiated, and therefore constitute a barrier to apoplastic ion flow only in root zones where an endodermis is already present. In a similar way to the endodermis, maturation of an exodermis involves further deposition of suberin and cellulosic wall material, further impeding flow of solution through walls.
The exodermis is not totally impermeable to water or nutrients and may have a differential selectivity that varies with the environment. How much the root is sealed off depends on the type of exodermis: (1) uniform exodermis where the cells are uniform in shape (suberin deposition is patchy and develops late) or (2) dimorphic exodermis, which consists of long and short passage cells (the former of which are suberised). Suberin lamellae enclosing the long cells disrupt the cytoplasmic continuity through plasmodesmata and the cell dies, but suberin lamellae in the uniform-type exodermis do not affect plasmodesmata. Individual passage cells allow passage of solutes and water via uptake carriers, not via the apoplast as flow there is blocked by the Casparian strip. They have an active role in ion uptake and often become the only plasmalemma facing the soil solution especially when the epidermis dies. Some families (e.g. irises) have large numbers of passage cells while others have very few.
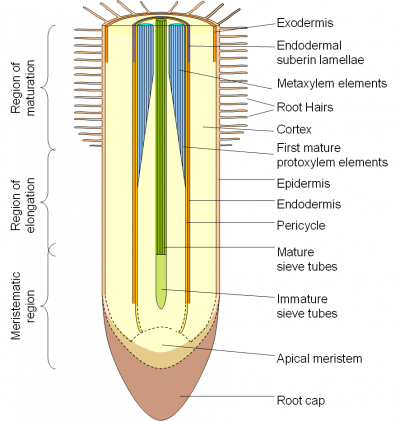
The diagram of a root to the right (Figure 3.54) indicates the stage of development at which the various structures are functional.
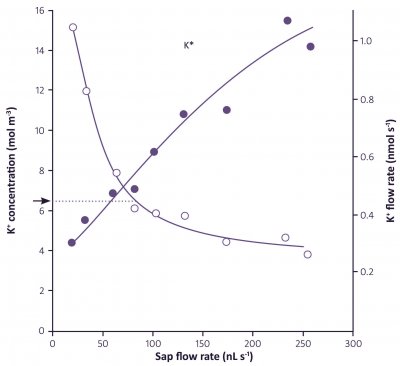
The anatomy of roots and the alternative pathways of water and solute flow across roots and into the xylem indicates that the relationship between water and nutrient flow to the shoots could be quite complex. Water may take different pathways than nutrients. The distribution of water in the different pathways depends not only on the age of the root, but may also vary with the flux of water moving through, that is, the rate of transpiration.