Since phloem translocation is confined to sieve elements embedded within a tissue matrix, it is difficult to obtain uncontaminated samples of translocated sap. The least equivocal approach has been to take advantage of the high P of sieve tube contents. Puncturing or severing sieve tubes should cause exudation of phloem sap provided a sealing mechanism is not activated.
For some plant species, sieve-pore sealing develops slowly, or can be experimentally down-regulated by massage or repeated excisions (Milburn and Kallarackal 1989) or slowed by puncturing the vasculature while it is snap frozen in liquid N2 (Pate et al 1984). Carefully placed incisions that do not disturb the underlying xylem, which in any case is more likely to be under tension, permit collection of relatively pure phloem exudate through the severed sieve tubes. Nevertheless, contamination with the contents of cells other than sieve tubes damaged at the site of incision is inevitable. For the major solutes of phloem such as sugars or amino acids that are present in high concentrations this problem is minimal but for less abundant molecules like hormones or other signals, particularly proteins or nucleic acids, conclusions about the origin and functions of these must be made with caution. The ‘natural hemophiliacs’ of the plant world are few and include a number of cucurbits, some brassicas, castor bean, species of the genus Yucca and some species of lupin (Lupinus albus, L. angustifolius, L. mutabilis and L. cosentinii). The excision technique has been expanded to plant species that do not readily exude, by chemically inhibiting the sealing mechanism. Callose production is blocked when wounded surfaces are exposed to the chelating agent ethylenediaminetetraacetic acid (EDTA) by complexing with calcium, a cofactor for callose synthase. Immersing whole, excised organs in EDTA solution, which is essential to inhibit blockage, risks contaminating sap with solutes lost from the apoplast as well as non-conducting cells. This is not an ideal technique.
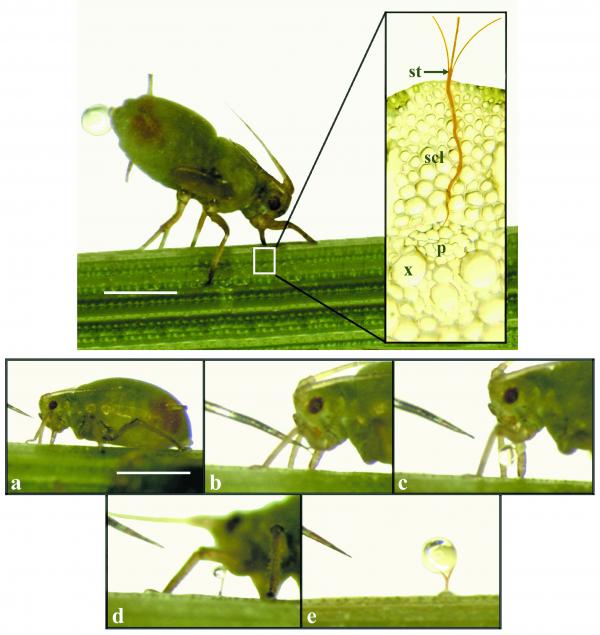
Enlisting sap-sucking aphids or leaf hoppers to sample sap has been more successful. Aphids can guide a long syringe-like mouthpart (a stylet) into conducting sieve elements (Figure 5.8). Pressure normally forces sieve-tube sap through the stylet into the aphid’s gut where it becomes food or is excreted as ‘honeydew’. By detaching the aphid from its mouthpart pure phloem contents can be collected from the cut end of the implanted stylet. Detaching the aphid body can be achieved by surgery following rapid anesthesia in high CO2 or by severing the stylet using a laser. While stylectomy has been successful with a number of monocotyledons (rice, wheat and barley) the technique has proved more difficult to use with dicotyledons, yielding at best a few microlitres of phloem contents. On the other hand collection of milliliter volumes of exudate from one of the natural hemophiliacs is possible permitting extensive analysis of solutes and macromolecules. In the case of lupins, exudation occurs readily at many sites on the plant so that solutes translocated from source tissues as well as entering sinks can be collected and analysed (Figure 5.9).