Balsamo RA, Uribe EG (1988) Plasmalemma- and tonoplast-ATPase activity in mesophyll protoplasts, vacuoles and microsomes of the Crassulacean-acid-metabolism plant Kalanchoe daigremontiana. Planta 173: 190-196
Borland AM, Dodd AN (2002) Carbohydrate partitioning in crassulacean acid metabolism plants. Funct Plant Biol 29: 707-716
Borland AM, Zambrano VAB, Ceusters J et al. (2011) The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytol 191: 619-633
Chinnock RJ (2015) Feral opuntioid cacti in Australia. The State Herbarium of South Australia, Adelaide.
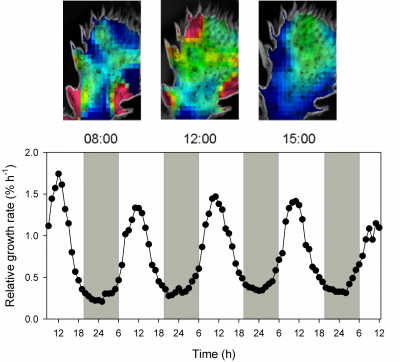
Gouws LM, Osmond CB, Schurr U et al. (2005) Distinctive diel growth cycles in leaves and cladodes of CAM plants: differences from C3 plants and putative interactions with substrate availability, turgor and cytoplasmic pH. Funct Plant Biol 32: 421-428
Griffiths H, Cousins AB, Badger MR et al. (2007) Discrimination in the dark: resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase during the crassulacean acid metabolism cycle. Plant Physiol 143: 1055-1067
Harvey A (2009) Draft state opuntioid cacti management plan. Government of South Australia, Adelaide.
Holtum JAM, Smith JAC, Neuhaus HE (2005) Intracellular transport and pathways of carbon flow in plants with crassulacean acid metabolism. Funct Plant Biol 32: 429-439
Keeley JE (2014) Aquatic CAM photosynthesis: a brief history of its discovery. Aquatic Bot 118: 38-44
Lüttge U (2002) CO2 concentrating: consequences in crassulacean acid metabolism. J Exp Bot 53: 2131-2142
Osmond CB, Neales T, Stange G (2008) Curiosity and context revisited: crassulacean acid metabolism in the Anthropocene. J Exp Bot 59: 1489-1502
Sage R (2014) Photosynthetic efficiency and carbon concentration in terrestrial plants: the C4 and CAM solutions. J Exp Bot 65: 3323-3325
von Caemmerer S, Griffiths H (2009) Stomatal responses to CO2 during a diel crassulacean acid metabolism cycle in Kalanchoe daigremontiana and Kalanchoe pinnata. Plant Cell Environ 32: 567-576
Walter A, Christ MM, Rascher U et al. (2008) Diel leaf growth cycles in Clusia spp. are related to changes between C3 photosynthesis and crassulacean acid metabolism during development and water stress. Plant Cell Environ 31: 484-491
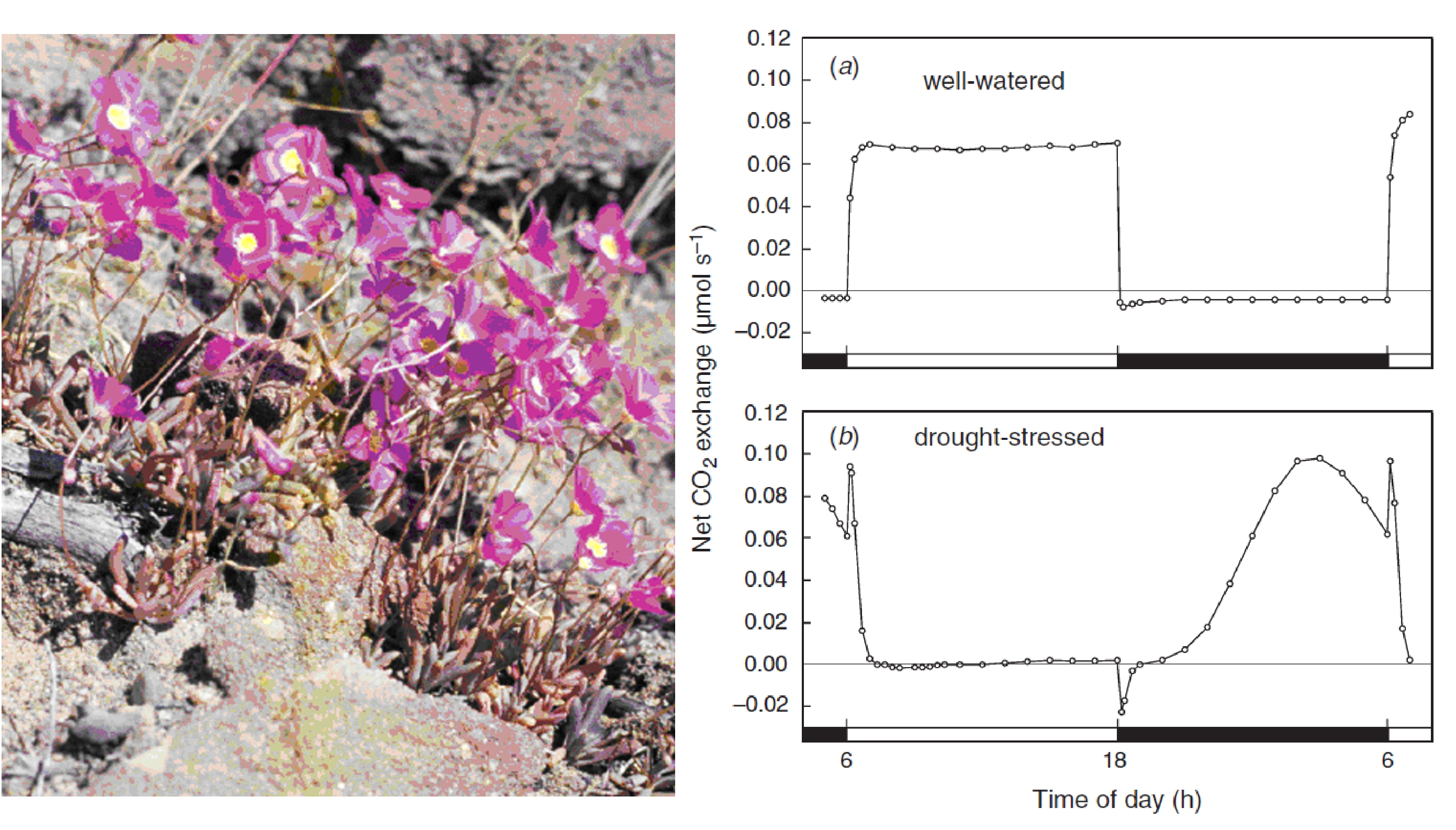
Winter K, Holtum JAM (2011) Induction and reversal of crassulacean acid metabolism in Calandrinia polyandra: effects of soil moisture and nutrients. Funct Plant Biol 38: 576-582
Winter K, Holtum JAM (2014) Facultative crassulacean metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. J Exp Bot 65: 3425-3441
Winter K, Aranda J, Holtum JAM (2005) Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Funct Plant Biol 32: 381-388
Winter K, Garcia M, Holtum JAM (2008) On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë and Opuntia. J Exp Bot 59: 1829-1840
Winter K, Holtum JAM, Smith JAC (2015) Crassulacean acid metabolism: a continuous or discrete trait? New Phytol 208: 73-78
Yang X, Cushman JC, Borland AM et al. (2015) A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol 207: 491-504
Zambrano VAB, Lawson T, Olomos E et al. (2014) Leaf anatomical traits which accommodate the facultative engagement of crassulacean metabolism in tropical trees of the genus Clusia. J Exp Bot 65: 3513-3523