Fig_p_5.26.png
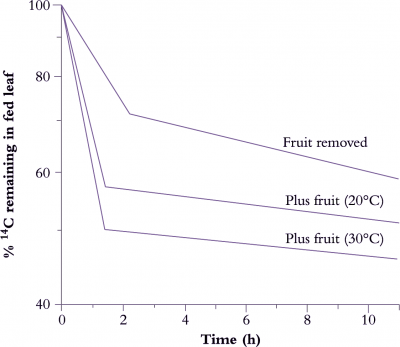
Figure 5.17. Time-course of photoassimilate export from source leaves of tomato plants. Control plants, in which fruits were a major sink for photoassimilates, were maintained at 20°C. Treatments involved (1) removing fruit or (2) exposing plants with fruits to 30°C. The proportion of 14C label remaining in source leaves after a radioactive pulse was monitored through time to show that (1) presence of major sinks or (2) more rapid metabolism accelerated 14C export from source leaves (Based on Moorby and Jarman 1975).
(a) Sink effects on export
The response of photoassimilate export to changes in sink demand depends upon whether photoassimilate flow is source or sink limited (Wardlaw 1990). A source-limited system does not respond rapidly to an increase in sink demand, depending more on the capacity of leaves to increase the size of the transport pool. In contrast, alterations in sink demand in a sink-limited system elicit immediate effects on photoassimilate export. Figure 5.17 shows how the presence of fruits accelerates 14C export, especially at high temperatures. For leaves that load the se–cc complexes from apoplasmic pools, changes in sink demand probably influence photoassimilate export by altering membrane transport properties. These changes in membrane transport entrain a flow of adjustments in biochemical partitioning within the leaf through substrate feedback (see below).
(b) Sink effects on membrane transport
Changes in the turgor pressure of phloem sap or altered phytohormone levels could serve as signals for sink demand.
Changes in the pressure of sink phloem sap are rapidly transmitted through sieve tubes to sources. Phloem loading in source tissues responds to this pressure signal by changes in solute transport rates mediated by membrane-associated porters (van Bel 1993). This is a proposed mechanism for phloem loading which would respond rapidly (within minutes) to changes in sink demand.
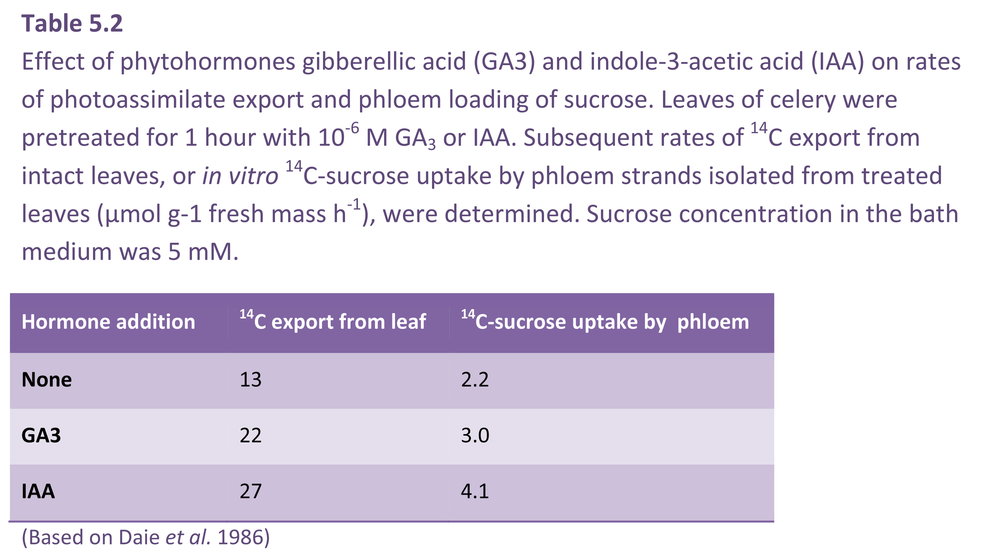
Phytohormone levels in leaves respond to changes in the source/sink ratio. For instance, gibberellin levels in leaves proximal to developing inflorescences increase at fruit set. In contrast, abscisic acid levels in soybean and grape leaves are inversely related to alterations in sink demand (Brenner 1987). Therefore, changes in leaf phytohormone levels could serve to signal shifts in sink demand for photoassimilates. In this context, direct application of auxin and gibberellic acid to source leaves results in a rapid enhancement of photoassimilate export (Table 5.2). Gibberellic acid did not stimulate leaf photosynthesis or alter photoassimilate partitioning, appearing instead to upregulate phloem loading. This was confirmed by faster 14C loading into isolated phloem strands (Table 5.2).
(c) Sink influences on biochemical partitioning within source leaves
A substrate feedback response is elicited if the rate of photo-assimilate export from chloroplasts is limited by sink demand. If sucrose export from source pools is accelerated by phloem loading, substrate feedback inhibition of photoassimilate delivery is alleviated. A cascade of adjustments in the activity of key regulatory enzymes follows (see Section 2.3) with the final outcome of an increased flow of sucrose into transport pools. Conversely, if photoassimilate flow is limited by photosynthetic rate, the activity of enzymes responsible for sucrose biosynthesis is not subject to feedback inhibition by substrates. As a consequence, responses to increased sink demand can only be mediated by increases in photosynthetic enzyme activity.