Plants and many algae contain two distinct protein complexes for trapping and processing photons of light; photosystems I and II (PSI and PSII). These two systems can be separated and identified using a combination of biochemical and chemical techniques. Within the chloroplast, however, these two systems must work cooperatively and sequentially to absorb photons and convert their quantum energy into a flow of electrons. Interestingly, although PSI was discovered first, in cyanobacteria, photosynthetic electron flow is initiated in PSII and then proceeds to PSI. In PSII electrons are provided through the splitting of water molecules. PSI is responsible for finally delivering these electrons to NADH+.
This section presents a historical account of the discovery of the two photosystems and how they work together to split water and produce NADH+.
Prior to the advent of high-precision leaf gas exchange methods (as employed for Figure 1.10), O2 evolution was taken as a measure of photosynthetic activity. Action spectra were measured on a number of plants and algae over the range of visible radiation. A crucial and consistent observation was that O2 evolution dropped off much faster in the long-wavelength red region (>690 nm) than did absorption. Put another way, more quanta were being absorbed at longer wavelengths than could be used for photosynthesis. It seemed at these longer wavelengths as though a light absorber was being robbed of energy-processing capacity.
Anticipating that bimodal absorption implied a two-step process, and knowing that chlorophyll also absorbed photons at shorter wavelengths, Robert Emerson (working at Urbana in the mid-1950s) supplemented far-red light with shorter wavelength red irradiance and demonstrated that the relatively low photosynthetic rate in far-red light could be significantly increased. In fact the photosynthetic rate achieved with the two light qualities combined could be 30–40% higher than the sum of the rates in far-red or shorter red when measured separately (Emerson et al. 1957). This phenomenon became known as the ‘Emerson Enhancement Effect’ and contributed to a working hypothesis for photosynthetic energy conversion based upon two photochemical acts (proposed by Duysens et al. 1961), but additional lines of evidence were impacting on this outcome.
At about the same time as Emerson was establishing his enhancement effect, Myers and French observed ‘sequential enhancement’; that is, a disproportionate increase in photosynthetic rate or efficiency when the two light qualities were separated in time. The upper limits of dark intervals between two flashes of different light quality were 6 s for far-red after green and 1 min for green after far-red. Clearly, the ‘product’ of photochemical act 1 was stable for 1 min, that of act 2 for only 6 s. This discovery implied that chemical intermediates, rather than an altered physical state, were involved in a two-step cooperation (see Clayton 1980).
According to physical laws of photochemical equivalence, there should be a 1:1 yield in converting light energy to chemical energy by a perfect system. Quantum requirement for such events would be 1. However in photosynthesis the absolute quantum requirement for O2 is much greater than I. In the 1950s, Robert Emerson (at Urbana) and co-workers determined that 8-10 quanta were required. Hill and Bendell (1960) suggested a 'Z' scheme that was consistent with a requirment of 8-10 quanta, the cooperation of 2 quanta in the separation of one strong reducing and one strong oxidising equivalent, and the operation of two sequential photochemical acts. Figure 1.11 is a greatly developed version of their original model.
Figp1.10-p.png
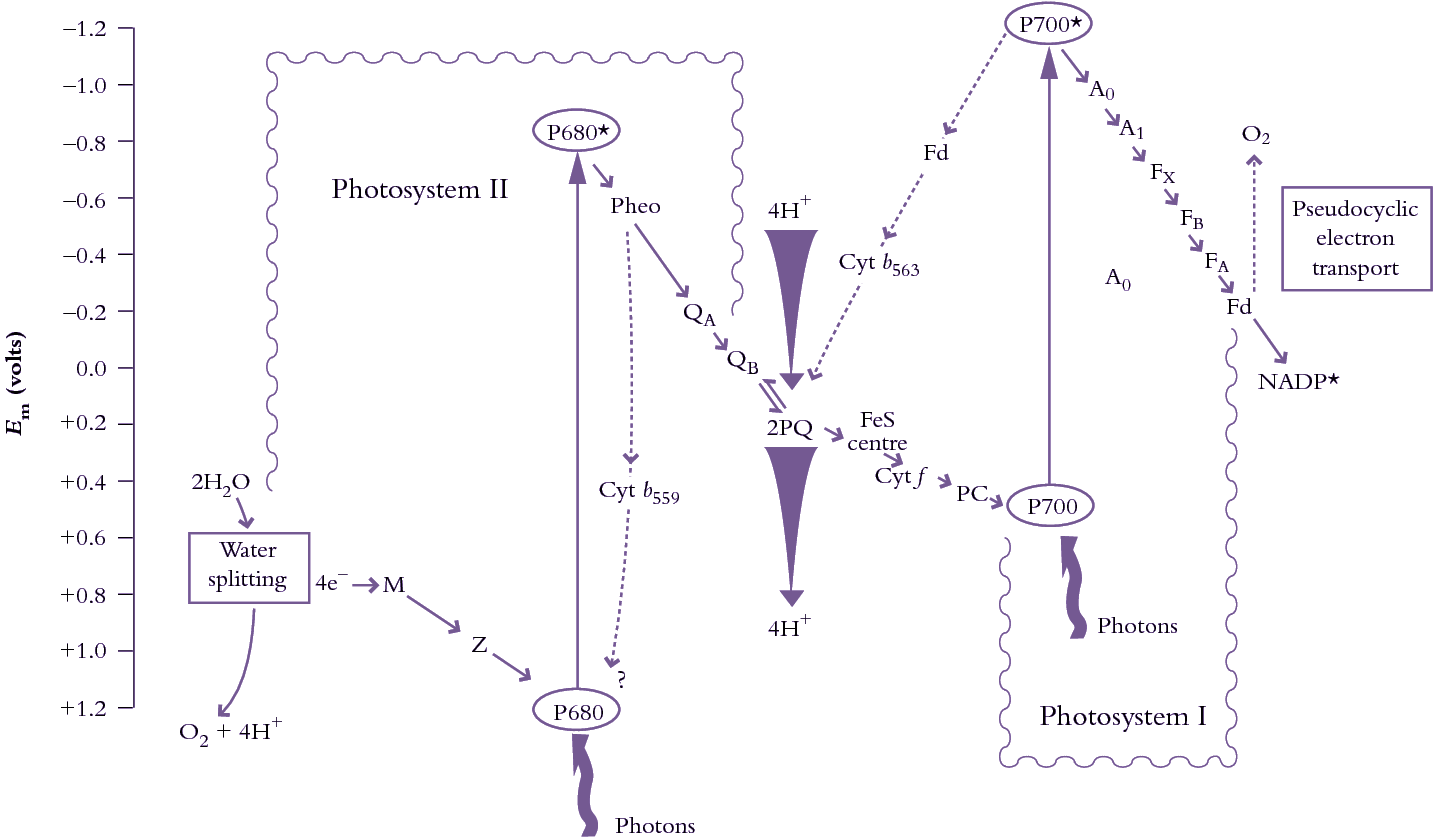
Figure 1.11. A highly diagrammatic zig-zag or ‘Z’ scheme of photosynthetic electron transport from water to NADP+ showing the sequence of electron/proton carriers and their association with either PSII or PSI. Linear electron flow is shown as solid lines; cyclic electron flow is indicated by dashed lines. All of these electron transport chains operate within thylakoid membranes with electron flow following a sequence dictated by redox potential (shown in volts on the ordinate). Cyclic electron flow in PSII diverts electrons from pheophytin to cytochrome b559 (and possibly back to P680+). Cyclic electron transport around PSI moves electrons from ferredoxin through cytochrome b565 and plastoquinone (PQ), while pseudocyclic electron transport takes electrons from ferredoxin to O2. (Original drawing courtesy C. Critchley).
In linear flow, water molecules are split in PSII, liberating O2 and providing a source of electrons. M is the manganese—containing cluster which oxidises water, Z is tyrosine-161 of the D1 protein which in turn represents the primary electron donor to P680+ (a special pair of Chl a molecules with an absorption peak at 680 nm). Pheo is the primary electron acceptor pheophytin a, a chlorophyll molecule lacking magnesium; QA is the first stable and permanently bound plastoquinone electron acceptor; QB is the second, temporarily bound, plastoquinone electron acceptor which actually leaves PSII in a reduced form (PQH2). Further along, FeS = Rieske iron—sulphur centre; Cyt f = cytochrome f; PC = plastocyanin; P700 = reaction centre chlorophyll a of PSI; A0, A1, FX, FB and FA are electron acceptors of PSI; Fd = ferredoxin; Cyt b559 = cytochrome b559; Cyt b563 = cytochrome b563. Also shown as tapered arrows is H+ accumulation in the lumen associated with water and plastoquinol oxidations.
The original version of this ‘Z’ scheme was further validated by unequivocal evidence from Australia that the two (inferred) photosystems were indeed separate physical entities. Using sophisticated biochemical chloroplast purification and subfractionation methods, coupled with detergent solubilisation of membranes, Boardman and Anderson (1964) achieved the first physical separation of photosystem II (PSII) and photosystem I (PSI), thus confirming the separate identities of those complexes.
A source of electrons had long been recognised as basic to the operation of this ‘Z’ scheme, with H2O molecules an obvious source, but were photosynthetic membranes capable of photolysis? Early experiments by Robin Hill and colleagues at Cambridge had established this capability. They used isolated thylakoid membrane preparations and showed that O2 could be evolved in the absence of CO2 as long as external electron acceptors were present (Hill reaction). Intact leaves or whole chloroplasts have no need for an artificial acceptor because electron flow is directed to NADP+ and subsequent reduction of CO2 (first demonstrated with intact chloroplasts; see Arnon 1984). The O2-evolving function of photosynthesis was found to be associated with PSII in experiments with isolated thylakoids using external (artificial) electron donors and acceptors and specific electron transport inhibitors. As one outcome of those early Cambridge experiments, O2 evolution is now measured routinely in vitro (and in vivo on leaves) with O2 electrodes (Walker 1987).
Chloroplast structure and function is by now sufficiently well defined to consider photosynthetic electron flow in detail. Figure 1.11 applies equally well to vascular plants or to algae with oxygenic photosynthesis, where in either case two photosystems work cooperatively and sequentially in absorbing photons and converting their quantum energy into a flow of electrons. Paradoxically, convention has it that photosynthetic electron flow initiates in PSII and proceeds to PSI. PSII was so named because PSI had already been described in single-celled (prokaryotic) organisms and, owing to the rules of nomenclature, was accorded priority.
Both photosystems are large multi-subunit complexes, quite different structurally and functionally, and operating in series. In PSII, electrons are provided from a water-splitting apparatus via a manganese complex which undergoes oxidation from a valency state of +2 to +4. These oxidation states are made possible by P680+ (a special form of Chl a with an absorption peak at 680 nm). P680+ is a powerful oxidant generated by absorption of energy from a photon. P680 is referred to as a ‘special pair’ because it is a pair of Chl a molecules. Electrons from P680 pass to pheophytin (Pheo in Figure 1.11) and on to a bound quinone molecule, QA. From there a second transiently bound quinone, QB, receives two electrons in succession and requires protonation. The entire, fully reduced, quinone molecule leaves PSII and enters a plastoquinone pool (2PQ).
In PSI, absorption of quantum energy from a photon causes oxidation of P700, the PSI reaction centre equivalent of P680. In contrast to PSII, where electrons are drawn from a water-splitting apparatus, P700 accepts electrons from PC (reduced form PC– in Figure 1.12). Electrons then pass through three iron–sulphur (FeS) centres and out of PSI to ferredoxin (Fd). The reaction centre of PSI contains several proteins, but most of the electron transfer cofactors are bound to large heterodimeric proteins which in turn bind the inner Chl a antenna. The LHCI complex consists of possibly eight polypeptides of between 24 and 27 kDa which carry Chl a and Chl b plus carotenoids.
Figp1.11-p.png
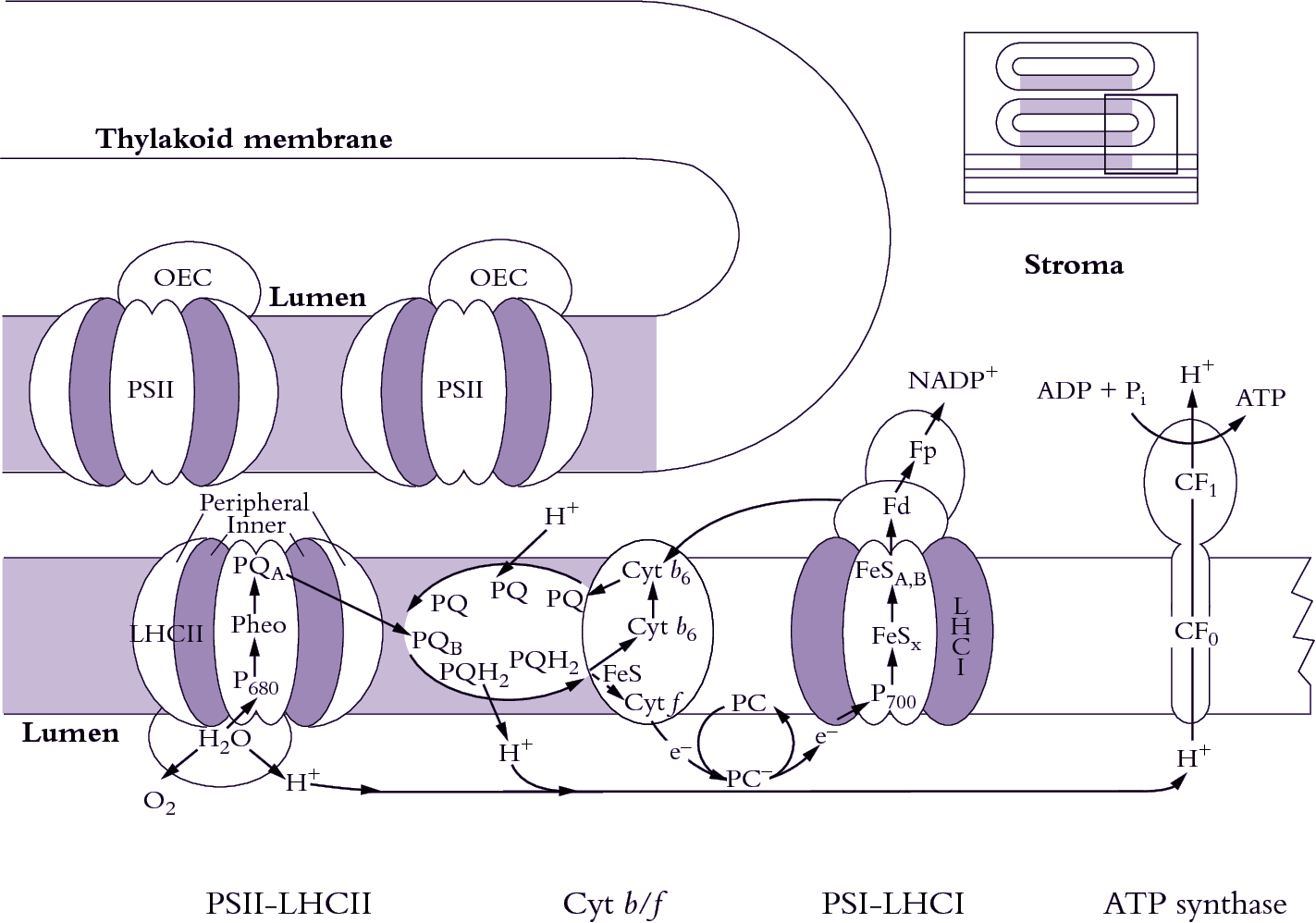
Figure 1.12. Light harvesting, photosynthetic electron transport from H2O to NADP+ and generation of ATP are achieved via four types of complexes which show a lateral heterogeneity within thylakoid membranes. A small part of a continuous network of interconnected thylakoids is shown here diagrammatically where PSI complexes and ATP synthase are restricted to non—appressed regions. Most PSII complexes and the light-harvesting assemblages associated with PSII (LHCII) are held within appressed regions of this network. Cytochrome b/f complexes (Cyt b/f) are more generally located. (Based on J.M. Anderson and B. Andersson, Trends Biochem Sci 13: 351-355, 1988)
A chemiosmotic coupling mechanism is responsible for ATP synthesis. Protons are ‘pumped’ across the thylakoid membrane from outside (stroma) to inside (lumen) by a complex arrangement of electron carriers embedded within the membrane. A prodigious concentration of protons builds up within the lumen, partly from photolysis of water molecules (water-splitting apparatus on PSII) and partly from oxidation of plastoquinone (PQ) on the inner face of the membrane. Hence, energy originally carried by incident photons is transduced into energy stored within an electrochemical gradient acrosss the thylakoid membrane. The protonmotive force from inside (lumen) to outside (stroma) is used to generate ATP within the stroma via an ATP synthase complex (CF0 and CF1) that straddles the thylakoid membrane. OEC = oxygen-evolving complex; Pheo = pheophytin a.These two photosystems are juxtaposed across thylakoid membranes in such a way that linear electron transport is harnessed for charge separation, leading to a massive accumulation of H+ ions within the lumen of illuminated thylakoids, which is then employed in ATP generation.
Combining concepts of photolysis and photosynthetic electron flow outlined earlier (Figure 1.11) and putting that conceptual framework into a thylakoid membrane system (Figure 1.12), a picture emerges where electrons generated from splitting H2O molecules on the inner surface of PSII are transferred from plastoquinol (PQH2) to the Rieske iron– sulphur centre (Rieske FeS) of the cytochrome b6/f complex (Cyt b6/f) and further to cytochrome f (Cyt f). The pivotal importance of Cyt f in facilitating electron transport from PSII to PSI was demonstrated by Duysens and colleagues (see Levine 1969), who showed that preferential energisation of PSII (light at <670 nm) caused reduction, whereas preferential energisation of PSI (light at >695 nm) caused oxidation. This elegant ‘push–pull’ experiment confirmed the cooperative and sequential nature of PSII and PSI, as well as indicating overall direction of photosynthetic electron flow.
Proteins which bind the Rieske FeS centre and Cyt f together with cytochrome b563 (Cyt b6) form a large electron transfer complex. This complex (Figure 1.12) spans the membrane and is located between the two photosystems. Electrons are transferred to PC (forming PC–), a copper-containing soluble protein extrinsic to the thylakoid membrane and located in the lumen. On the other side of the membrane, attached to the stromal side, is ferredoxin (Fd) which accepts electrons from PSI and passes them on to ferredoxin–NADP reductase, an enzyme, also extrinsic to thylakoids, and attached on the stromal side of the thylakoid membrane. This enzyme accomplishes the final electron transfer in an overall linear chain and reduced NADP is then protonated.
While linear electron transport from water to NADP+ is the main and most important path, electrons can also be transferred to O2 in a so-called pseudocyclic or Mehler reaction (Figure 1.11). This pathway probably operates in vivo as a sink for electrons when synthetic events call for more ATP than NADPH. Electrons can also be cycled around both PSII and PSI. Electrons cycling around PSI will produce ATP but with no accompanying NADPH. Cyclic electron flow around PSII may have a completely different role and may be related to the downregulation of this photosystem during photoinhibition (Chapter 12).
According to this multistage scheme, electrons are transferred from donor (reductant) to acceptor (oxidant). The direction of that transfer depends upon a difference in oxidation–reduction potential between a given donor and a given acceptor (as indicated on the ordinate in Figure 1.11). A more positive potential implies stronger oxidative power (i.e. capacity to accept electrons); a more negative potential implies stronger reducing power (i.e. capacity to donate electrons). P680* thus has a strong capacity to donate electrons (a strong reductant); P700* has an even stronger capacity to donate electrons (an even stronger reductant).
Molecules which accept electrons are immediately protonated. In aqueous systems, such as chloroplasts in vivo, hydrogen ions (H+) are ubiquitous, and these ions combine with electron acceptors to generate hydrogen atoms (i.e. H+ ion + electron → H atom). In Figure 1.11, some events involve electron transfer, while others include transfer of hydrogen atoms. As a simplifying convention, all such events are referred to as electron transfers. Ironically, the end result of all these reactions is a net transfer of hydrogen atoms!
