Plant and animal membranes have much higher permeability to water than can be explained by diffusion rates through a lipid bilayer. Furthermore, the activation energy for diffusion of water across a plant membrane is lower than would be expected across a lipid bilayer, where water has to overcome the high-energy barrier of partitioning into a very hydrophobic oily layer. Some reports put the activation energy for water flow across membranes as low as the value for free diffusion of water. In other words, water enters the membrane about as readily as it diffuses through a solution. This suggests that water is moving across the membrane through a pathway other than the lipid, perhaps some kind of water pore or water channel. Since the discovery of water channel proteins in animal cell membranes, molecular biologists discovered that similar proteins exist in plants.
Water channels, like ion channels, are proteins embedded in membranes that facilitate the passive transport (non-energised flow) of water or ions down their respective energy gradients. Movement of a solute or water through these transport proteins is not coupled to movement of any other solute, and does not require ATP. The proteins that facilitate passive transport are diverse; some are specific for particular ions and allow high transport rates per protein molecule (ion channels), some are specific for water (water channels or aquaporins) and some are specific for neutral solutes and may have slower transport rates per protein molecule.
Why are there water channels in membranes when the lipid itself is already somewhat permeable to water? There are several rationales for the presence of water channels in plant membranes. One is that specialised transport proteins can control water flow. That is, a water channel protein may be turned on and off, for example by phosphorylation, while water permeability of the lipid is essentially constant. In animal cells, such as in the kidney, water channels are controlled by antidiuretic hormones. Plant hormones could also influence the function of water channels. A second rationale for the presence of water channnels is to balance water flow and prevent bottlenecks. In the root, water channels are most abundent in the endodermis and inner stele where water flow across membranes is rapid.
The approach to studying water channels has been to inject genetic material from plants into Xenopus oocytes (a particular type of frog’s egg). The Xenopus oocyte is particularly useful because it is large, enabling observations of cell response to foreign proteins. It is one of several expression systems along with Chara (giant algal cells) and yeast cells. cDNA arising from screens of cDNA libraries can be injected into the Xenopus nucleus, or poly (A)+-RNA can be injected into the oocyte cytoplasm where it is translated. Plant water transport proteins expressed in the oocyte plasma membrane result in physiological changes; for example, the oocyte swells rapidly when the external osmotic pressure in the bathing medium is lowered (Figure 3.71a). The first plant aquaporin gTIP (Tonoplast Integral Protein) that was discovered occurs in the tonoplast and probably accounts for its high water permeability. Provided that the increase in water permeability is not a consequence of some other change or a side effect of other types of transport, it can be concluded that the protein catalyses transport of water across membranes.
3.0-Ch-Fig-3.71.png
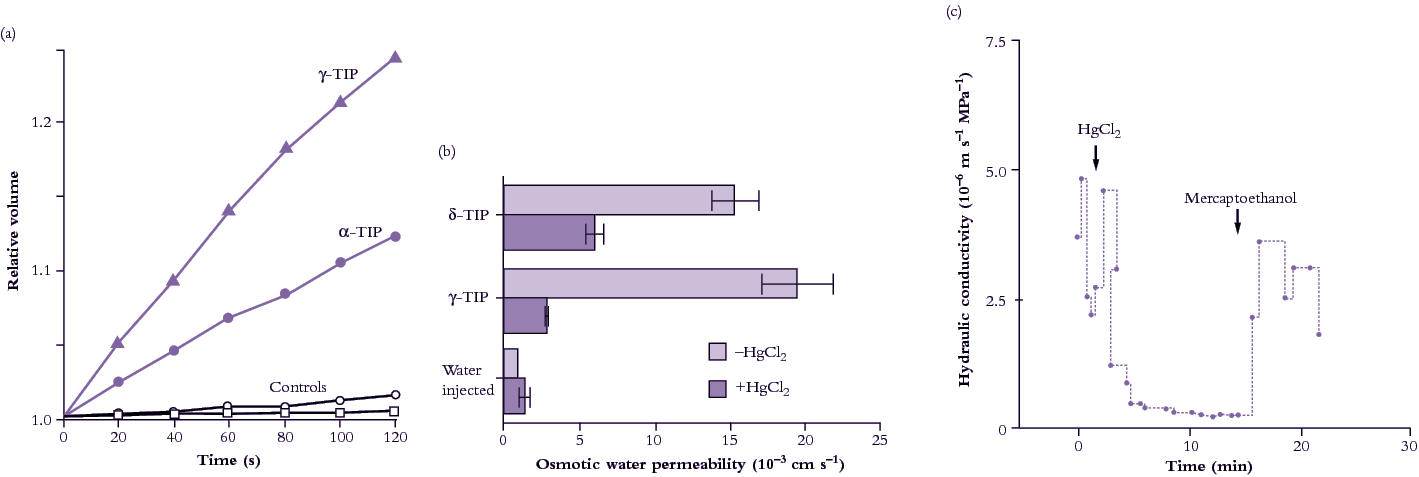
Figure 3.71 Evidence for presence of aquaporins (water channels) in plant membranes. (a) Change in volume of Xenopus oocytes injected with two TIP proteins after lowering osmotic pressure of the external medium (Maurel et al. 1995). (b) Sensitivity of two TIP proteins expressed in Xenopus oocytes to mercuric chloride (HgCl2), a general inhibitor of aquaporins (Daniels et al. 1996). (c) Inhibitory effect of HgCl2 on hydraulic conductivity of the freshwater alga Chara corallina measured with a pressure probe (Schütz and Tyerman 1997).
Water channels can be inhibited by mercuric chloride when expressed in Xenopus occytes (Figure 3.71b), and also in plant cells (Figure 3.71c). Inhibition is reversed by applying mercaptoethanol to block the action of HgCl2. Under mercury inhibition, the activation energy of water flow increases markedly indicating that water flow is now restricted to diffusional flow across the lipid bilayer, that is, aquaporins are blocked.
Plants have many aquaporin genes. For example, Arabidopsis thaliana has 35 and rice (Oryza sativa) has 33. Proteins encoded by aquaporin genes are localized in the plasma membrane, tonoplast and endo membranes and classed as Plasma membrane Intrinsic Proteins (PIPs), Tonoplast Intrinsic Proteins (TIPs), Nodulin26-like Intrinsic Proteins (NIPS), Small basic Intrinsic Proteins (SIPs) or X Intrinsic Proteins (XIPs) (Luu and Maurel 2013). Within membranes, clustering of PIPs in membrane rafts has been observed, and there is variation in the lateral mobility for different aquaporins. Of the many aquaporin proteins in plants, some transport water only (up to 109 molecules per second) and others are permeable to a range of neutral solutes such as gases (carbon dioxide, ammonia), metalloids (boron, silicon, arsenic), or reactive oxygen species (hydrogen peroxide). The transcription, translation, trafficking, and gating of PIPs are regulated by environmental and developmental factors involving signalling molecules, phytohormones and the circadian clock (Chaumont and Tyerman 2014). The transcription, translation, trafficking, and gating of PIPs are regulated by environmental and developmental factors involving signalling molecules, phytohormones and the circadian clock (Chaumont and Tyerman 2014).
When PIP genes are transcribed, their mRNA is translated in the rough endoplasmic reticulum (ER), and the proteins targeted to the plasma membrane. PIPs belonging to the PIP2 group form homo-oligomers, or hetero-oligomers by associating with PIP1 isoforms. Some PIP2s contain a diacidic motif in their N terminus that acts as an ER export signal. It may be recognized by Sec24 which is the main “cargo” selection protein of the coat protein complex COPII that mediates vesicle formation at the ER export sites. PIP oligomers then transit through the Golgi apparatus and trans-Golgi network and are then loaded into secretory vesicles and routed to the plasma membrane (Figure 3.72). Insertion of PIPs into the plasma membrane is mediated by a protein that regulates vesicle fusion (the “syntaxin” SYP121). The plasma-membrane localized PIPs can be recycled internally: once internalized in vesicles, PIPs are delivered to the trans-Golgi network before being routed back to the plasma membrane or directed into lytic vacuoles for degradation (Figure 3.72). Salt stress causes dephosphorylation and internalization of PIPs, and drought stress induces ubiquitylation of PIPs, which are then degraded in the proteasome.
Aquaporins assemble as homo- or hetero-tetramers, each monomer acting as an independent water channel. The structure of an aquaporin monomer (Figure 3.72) consists of six membrane-spanning α-helices connected by five loops, with both N and C termini facing the cytosol. Two loops form two short hydrophobic α-helices dipping halfway into the membranes, which, together with the membrane-spanning helices, create a pore with high specificity (Murata et al. 2000).
3.0-Ch-Fig-3.72.png
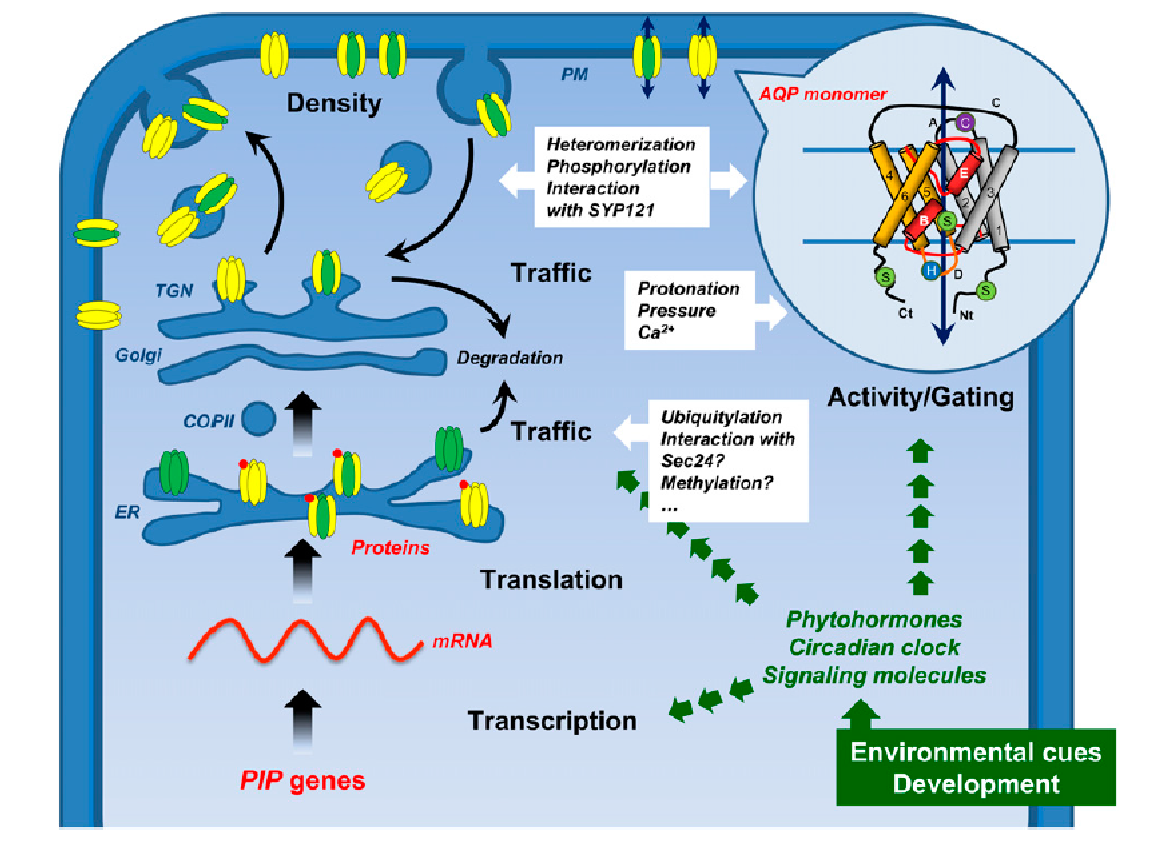
Figure 3.72 Formation and trafficking of PIPs within a plant cell. PIPs belonging to the PIP2 group (in yellow) form homo- or heterooligomers by associating with PIP1 isoforms (in green). PIP oligomers transit through the Golgi apparatus and trans-Golgi network (TGN) and are then loaded into secretory vesicles and routed to the plasma membrane. In the circle is shown the topological structure of the aquaporin monomer AQP1 (Murata et al. 2000), with six membrane-spanning α-helices (1-6) connected by five loops (A-E). The loops B and E form two short hydrophobic α-helices (in red) dipping halfway into the membranes, which, together with the membrane-spanning helices, create a pore with high specificity. From Chaumont and Tyerman (2014). Reproduced from F. Chaumant and S.D. Tyerman, Plant Phys 164: 1600-1618, plantphysiol.org. Copyright American Society of Plant Biologists.
Aquaporins play a central role in regulating plant water relations. Water diffusion across cell membranes is facilitated by aquaporins that provide plants with the means to rapidly and reversibly modify water permeability. This is done by changing aquaporin density and activity in the membrane, including post-translational modifications and protein interaction that act on their trafficking and gating. At the whole organ level aquaporins modify water conductance and gradients at key “gatekeeper” cell layers that impact on whole plant water flow and plant water potential. In this way they may act in concert with stomatal regulation.
PIP and TIP expression is higher during the day than the night, and correlates with diurnal changes in transpiration. It is likely under circadian regulation. Expression can correlate with changes in hydraulic conductivity, Lp, so that aquaporins are more abundant or have higher activity at times when stomatal conductance is higher. Over a diurnal cycle, Lp can change 2-5 fold and so can PIP transcript activity or protein abundance (Chaumant and Tyerman 2014). This occurs in both roots and shoots.
This section has shown how the flow of water and ions across membranes are linked. The function of membrane transport in regulating nutrient supply is covered in Chapter 4.