Brett J Ferguson and Peter M Gresshoff, Centre for Integrative Legume Research, University of Queensland, Australia
Forming nodules and supplying rhizobia with food for nitrogen fixation are energy demanding processes for the host plant. As a result, legumes have developed innate mechanisms to balance their need to acquire nitrogen with their ability to expend energy (reviewed in Ferguson et al. 2010; Reid et al. 2011).
Nitrogen availability: why form nodules when there is plenty of nitrogen available?
When ample nitrogen is available in the soil, legume plants require fewer nodules to meet their nitrogen demands. Accordingly, they have an inbuilt mechanism in the root to detect the nitrogen content of the surrounding soil. Nitrogen-based compounds, such as nitrate, trigger the production of a small, hormone-like peptide signal, called Nitrate-Induced CLE 1 (NIC1) in soybean. NIC1 is predicted to be perceived by the receptor, Nodule Autoregulation Receptor Kinase (NARK). This perception results in the production of a novel inhibitor signal that acts locally in the root to prevent further nodulation events.
Stress: nodulation is a luxury for a stressed plant.
Nodulation is reduced in plants experiencing stress. This likely helps the plant to conserve its resources for combating the stress and for all-important seed development. To date, a number of stress-related factors have been found to inhibit nodule formation locally in the root, including ethylene, salicylic acid and various reactive oxygen species (reviewed in Ferguson and Mathesius 2014). Acidic soil conditions also reduce nodulation, with low pH also causing elevated soil Al3+ levels that negatively affect root growth.
Autoregulation of nodulation: too much of a good thing is not good.
Less than 10% of rhizobia infection events result in the formation of a fully-developed nodule. This is largely due to the Autoregulation Of Nodulation (AON) mechanism that the host plant uses to control its nodule numbers (reviewed by Ferguson et al. 2010; Reid et al. 2011). Mutant legume plants lacking a functional AON pathway are unable to regulate their nodule numbers and as a result exhibit a supernodulation phenotype (i.e. they develop an excessive number of nodules, with up to 25,000 per plant scored, Figure 1). When these supernodulating mutants are induced to form nodules, they are typically reduced in stature and often yield about 20 – 30% less, likely a direct result of their resources being used to form excess nodule structures (Figure 1).
4.3-CS-Fig-1.png

Figure 1 Legume nodulation and autoregulation. A, Soybean plants grown in nitrogen-poor conditions. Wild-Type (WT) plants form functional root nodules when inoculated with compatible, nitrogen-fixing, Bradyrhizobium japonicum. Non-nodulating mutant plants are unable to form nodules (nod-) and supernodulating mutant plants (nod++) are unable to regulate the number of nodules they form. B, Roots of wild-type (WT) and supernodulating mutant (nod++) common bean plants exhibiting mature nodule structures.
4.3-CS-Fig-2.png
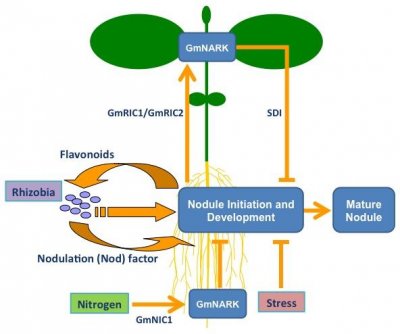
Figure 2 Regulation of legume nodule development. Legume roots exude flavonoid molecules, which attracts compatible rhizobia and triggers them to produce a Nod factor signal. Stress and Nitrogen locally inhibit nodule development. Nitrogen triggers the production of a CLE peptide, called GmNIC1 in soybean, that acts through the GmNARK receptor to suppress nodulation. The Autoregulation Of Nodulation (AON) acts systemically through the shoot. CLE peptides, called GmRIC1 and GmRIC2 in soybean, are produced in the root in response to the first nodulation events. These signals are transported to the shoot where they are perceived by GmNARK, which triggers the production of a shoot-derived inhibitor (SDI) signal that travels to the root to prevent further nodulation.
Although it has not yet been identified, SDI is reported to be a small, heat-stable, Nod factor- and NARK-dependent molecule that is not likely an RNA or protein (Lin et al. 2010) and has been recently been proposed to be the classical phytohormone, cytokinin (Sasaki et al. 2014). Additional factors acting downstream of SDI include Too Much Love, a Kelch-repeat transcription factor whose role in the AON process is yet to be fully defined.
An interesting finding is that of Wang et al. (2014), who suggest that the AON pathway may involve a microRNA after the tentative cytokinin signal. Specifically miR172c was shown to negatively regulate the transcript abundance of a gene (GmNNC1) encoding a transcription factor, being part of the AP2 family. GmNNC1 negatively targets the early nodulin gene ENOD40, needed for nodulation progress in soybean, Medicago truncatula, and Lotus japonicus. It now appears critical to connect the function of a peptide-activated receptor (GmNARK) with the reported cytokinin signal and the subsequent negative regulation cascade described by Wang et al. (2014).
References
Ferguson BJ, Indrasumunar A, Hayashi S et al. (2010) Molecular analysis of legume nodule development and autoregulation. J Integr Plant Biol 52: 61-76
Ferguson BJ, Mathesius U (2014) Phytohormone regulation of legume-rhizobia interactions. J Chem Ecol 40: 770-790
Lin Y-H, Ferguson BJ, Kereszt A, Gresshoff PM (2010) Suppression of hypernodulation in soybean by a leaf-extracted, NARK- and Nod factor-dependent small molecular fraction. New Phytol 185: 1074-1086
Okamoto S, Shinohara H, Mori T et al. (2013) Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nature Comms 4, doi:10.1038/ncomms3191
Ogawa-Ohnishi M, Matsushita W, Matsubayashi Y (2013). Identification of three hydroxyproline O-arabinosyltransferases in Arabidopsis thaliana. Nature Chem Biol 9: 726-730
Reid DE, Ferguson BJ, Hayashi S et al. (2011) Molecular mechanisms controlling legume autoregulation of nodulation. Ann Botany 108: 789-795
Sasaki T, Suzaki T, Soyano T et al. (2014). Shoot-derived cytokinins systemically regulate root nodulation. Nature Comms 5, doi:10.1038/ncomms5983.
Wang Y, Wang L, Zou Y et al. (2014) Soybean miR172c targets the repressive AP2 transcription factor GmNNC1 to activate GmENOD40 expression and regulate nodule initiation. Plant Cell 26: 4782–4801