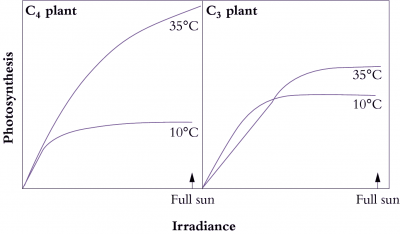
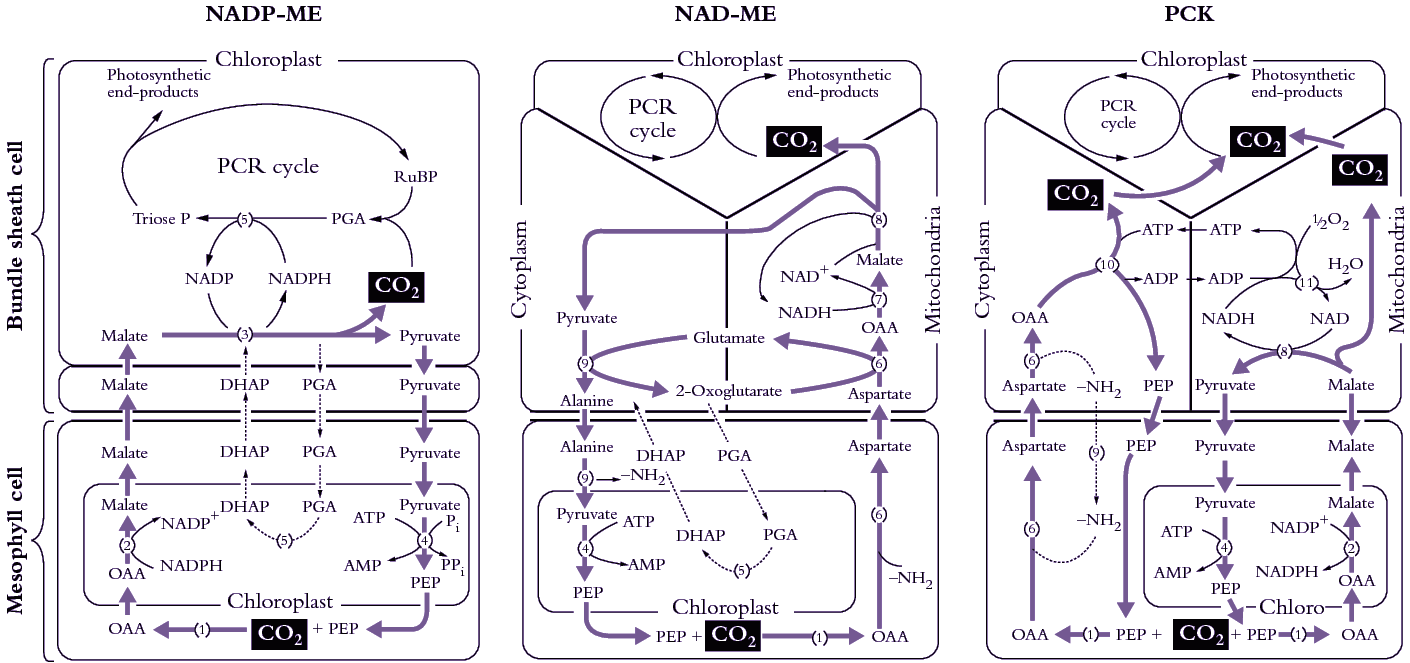
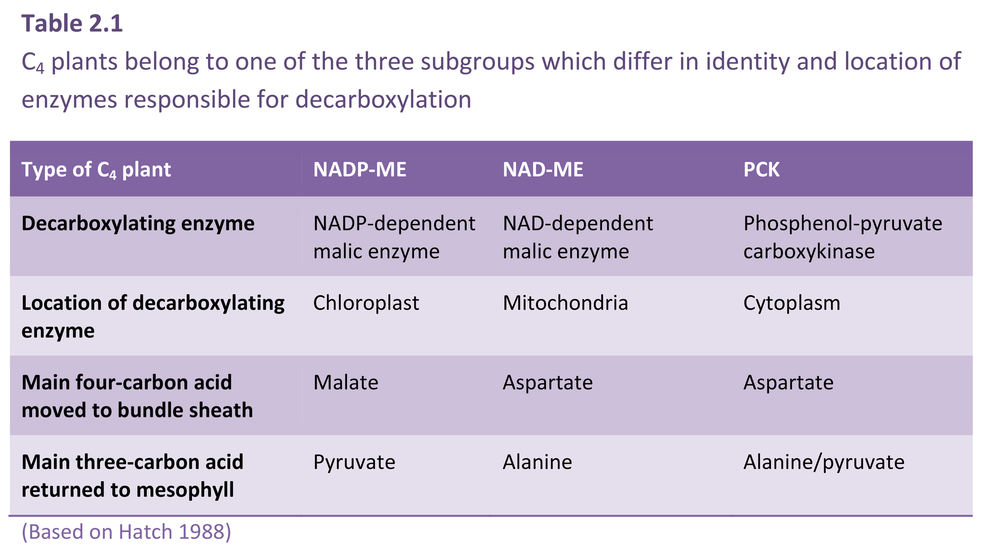
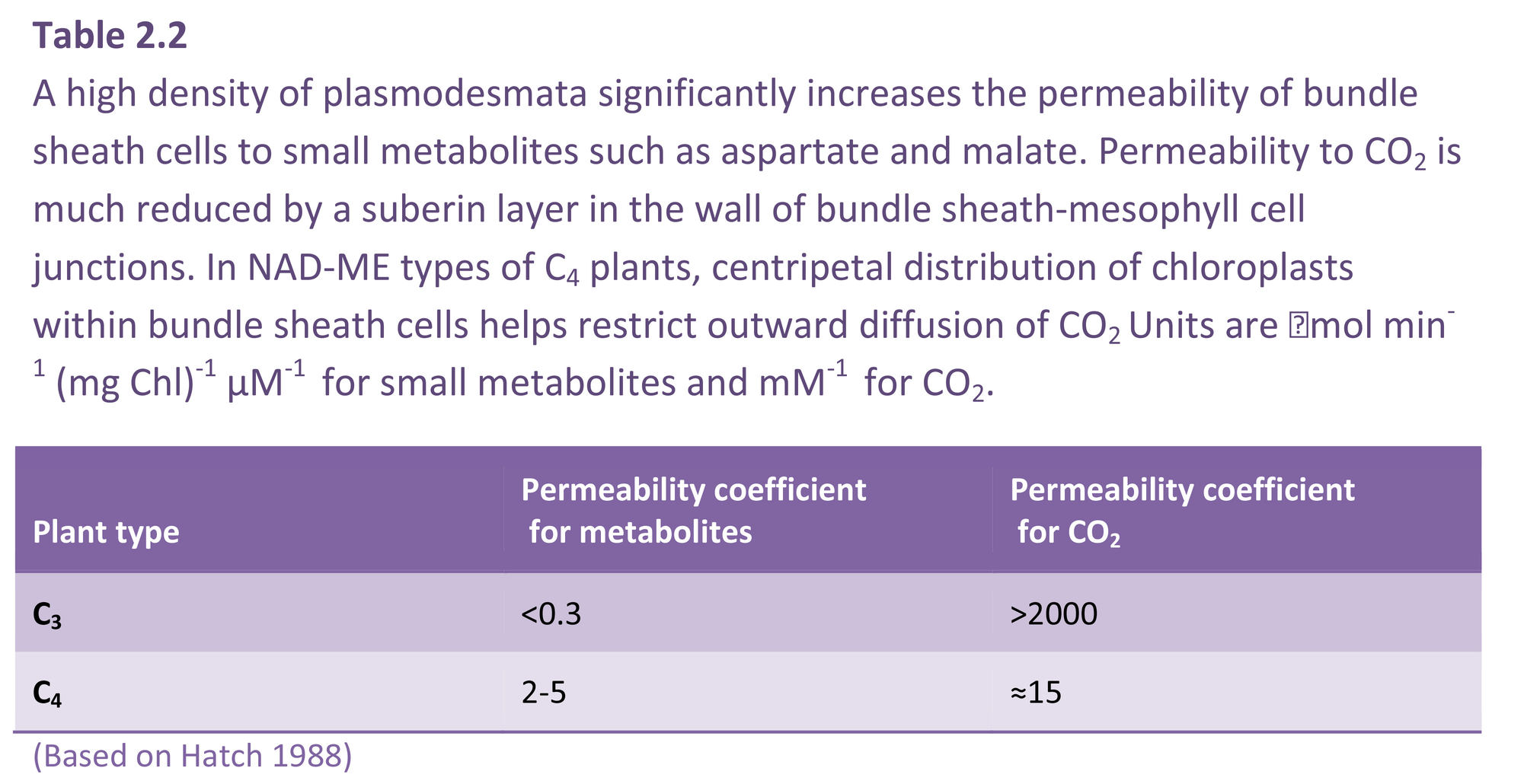
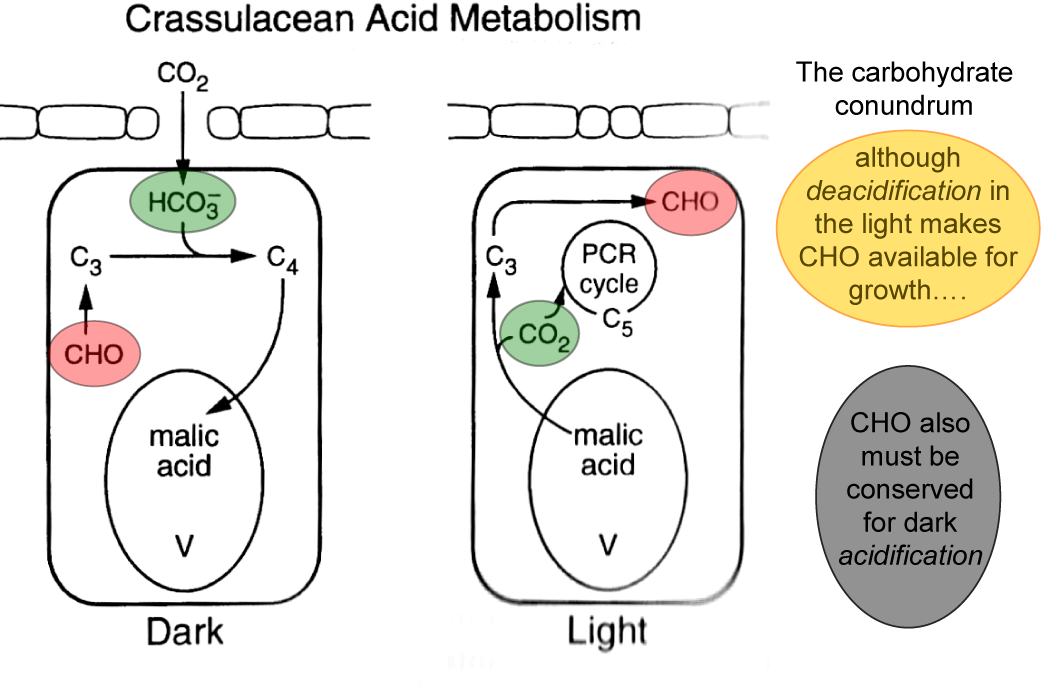
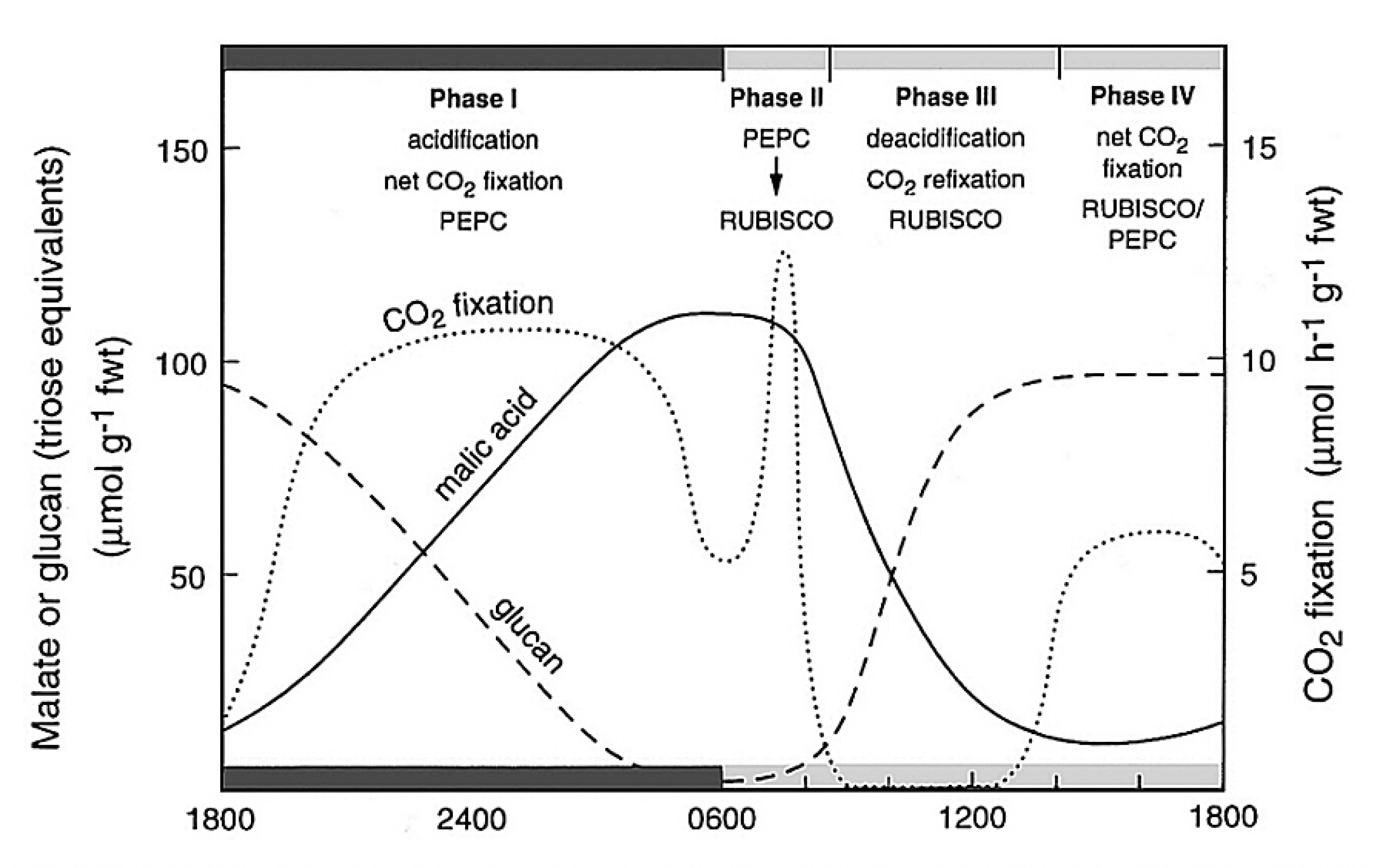
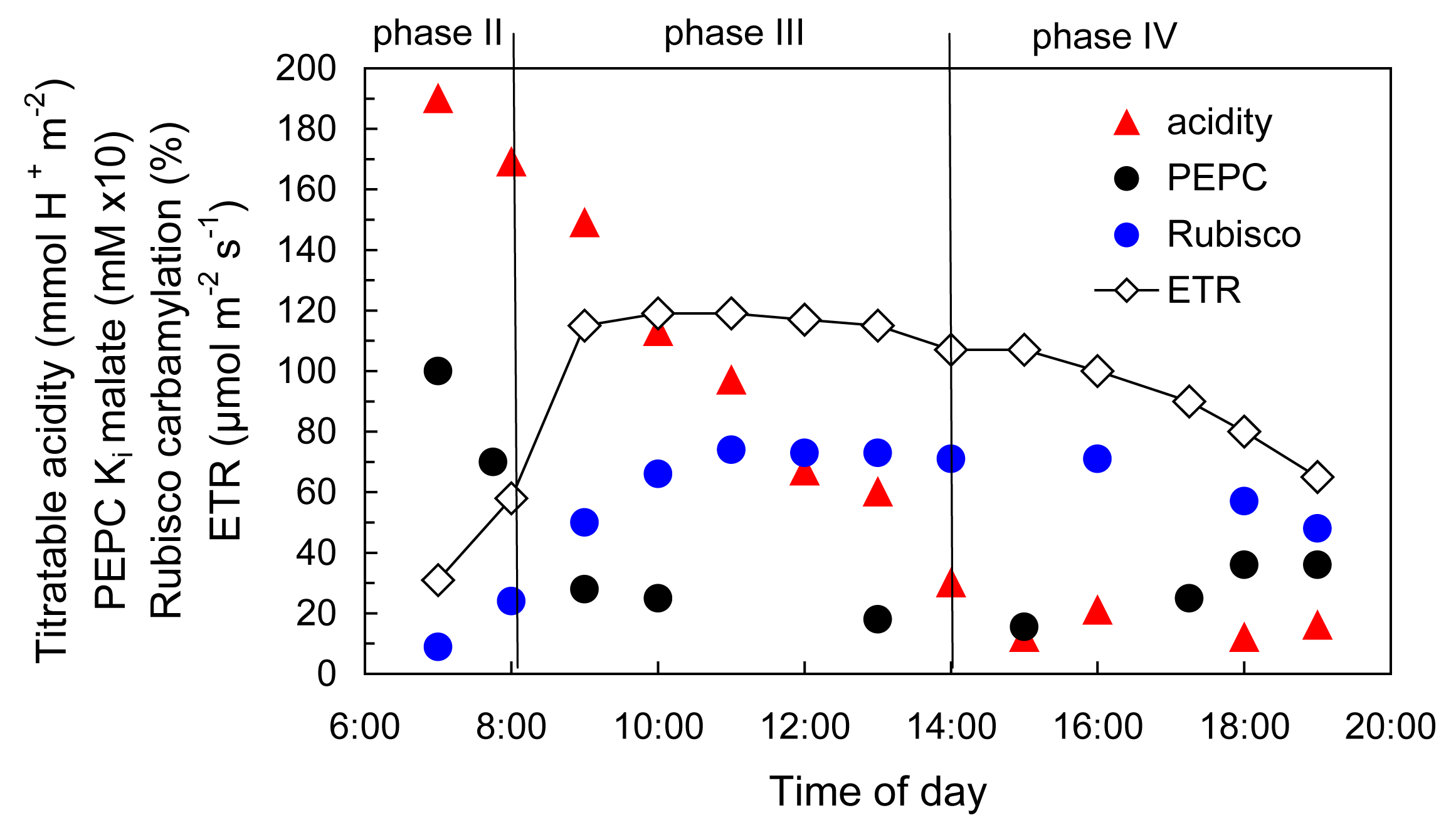
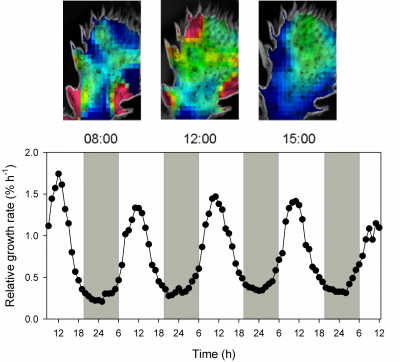
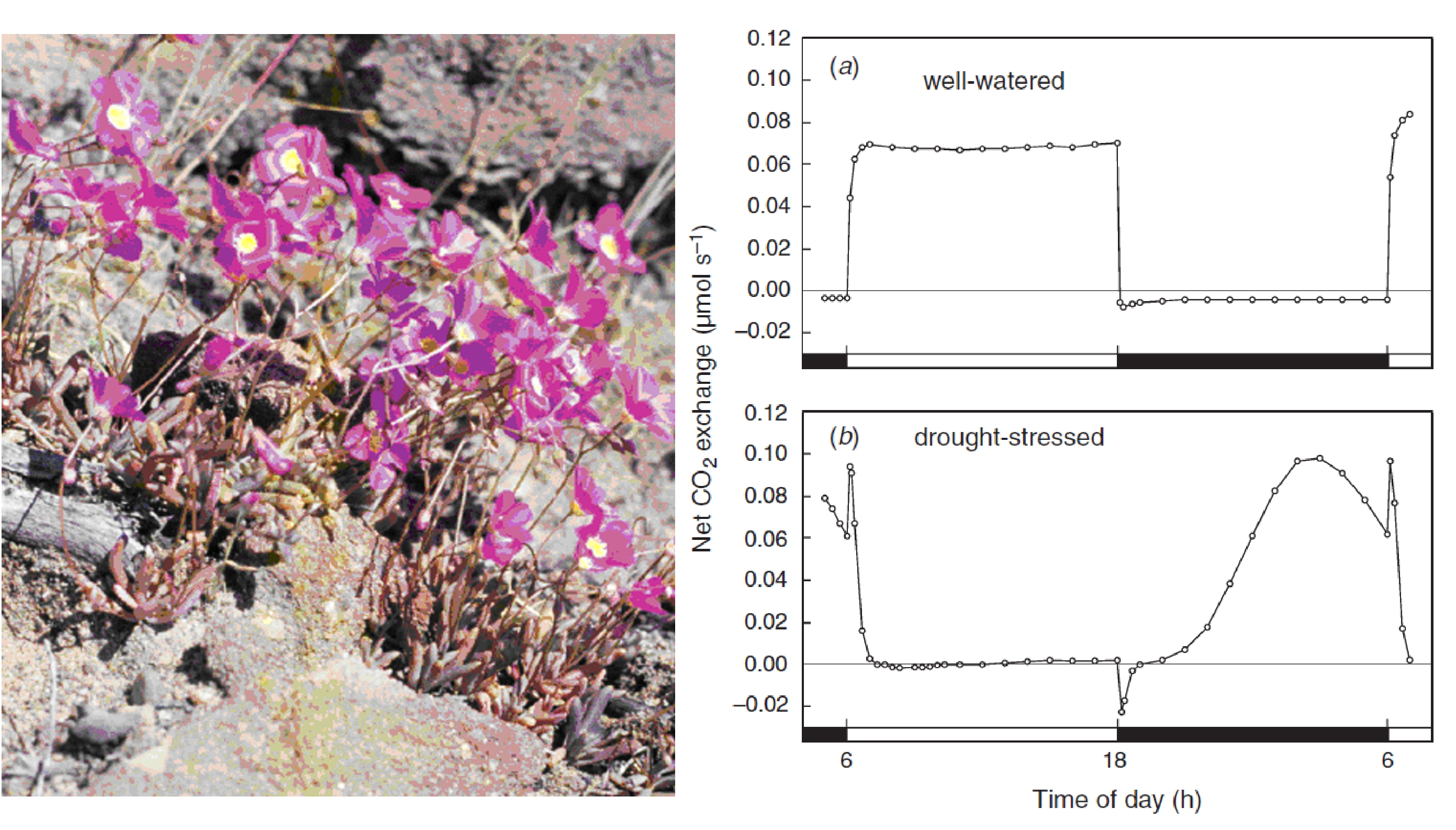
Bolton JK, Brown RH (1980) Photosynthesis of grass species differing in carbon dioxide fixation pathways. V. Response of Panicum maximum, Panicum milioides, and tall fescue (Festuca arundinacea) to nitrogen nutrition. Plant Physiol 66: 97-100
Brown DA (1980). Photosynthesis of grass species differing in carbon dioxide fixation pathways. IV. Analysis of reduced oxygen response in Panicum milioides and Panicum schenckii. Plant Physiol 65: 346-349
Christin PA, Samaritani E, Petitpierre B et al. (2009) Evolutionary insights on C4 photosynthetic subtypes in grasses from genomics and phylogenetics. Genom Biol Evol 1: 221–230
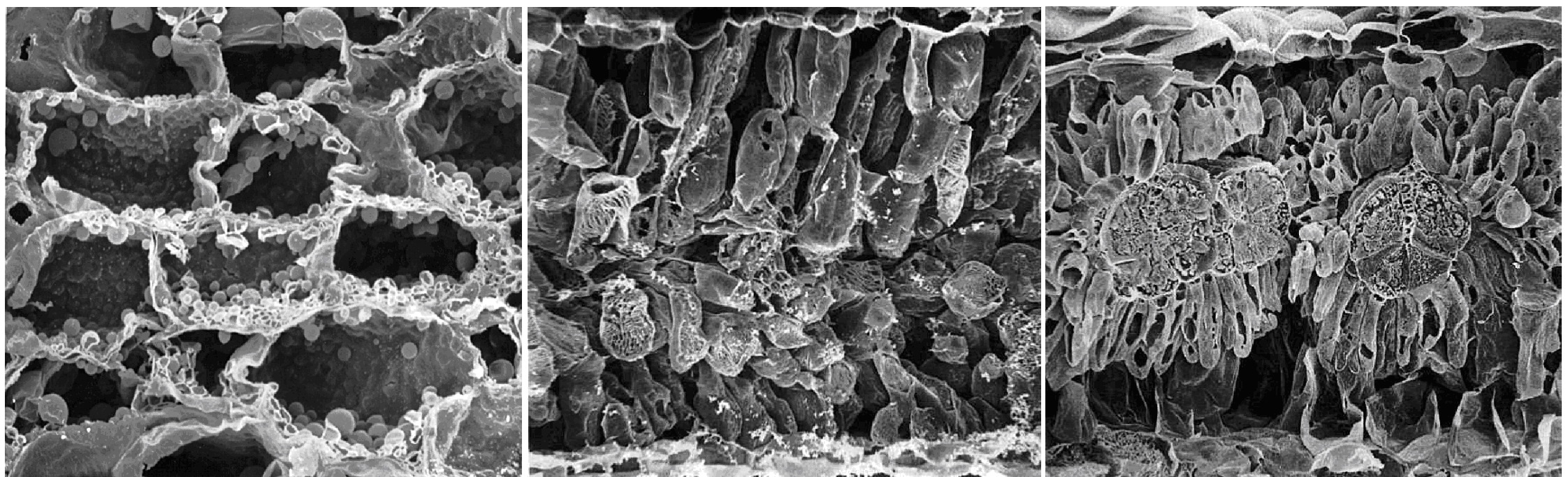
Edwards GE, Franceschi VR, Voznesenskaya EV (2004) Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55: 173–196
Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285-299
Ghannoum O, von Caemmerer S, Conroy JP (2002) The effect of drought on plant water use efficiency of nine NAD–ME and nine NADP–ME Australian C4 grasses. Funct Plant Biol 29: 1337-1348
Ghannoum O, Evans JR, Chow WS et al. (2005) Faster rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology 137: 638-650
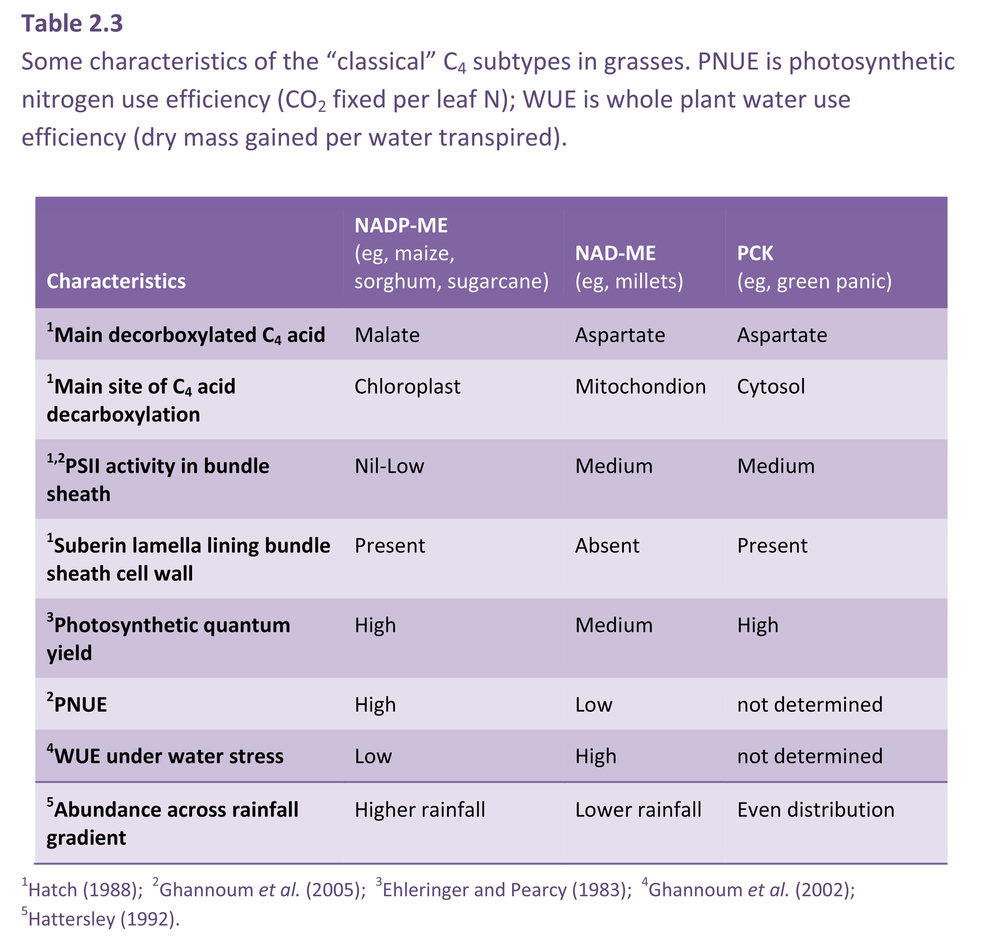
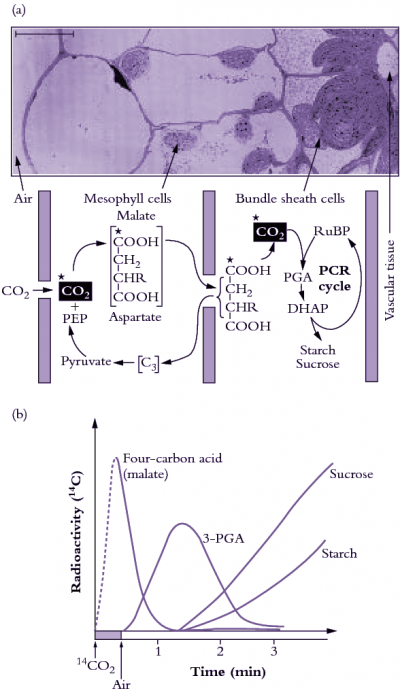
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106
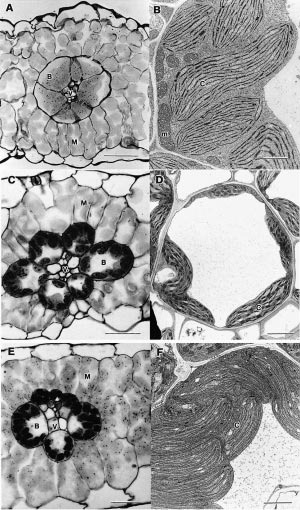
Hatch MD, Kagawa T, Craig S (1975) Subdivision of C4-pathway species based on differing C4 acid decarboxylating systems and ultrastructural features. Aust J Plant Physiol 2: 111-128.
Hattersley PW, Watson L, Osmond CB (1977) In situ immunofluorescent labelling of ribulose-1,5-bisphosphate carboxylase in leaves of C3 and C4 plants. Aust J Plant Physiol 4: 523-539
Hattersley PW (1992) In ‘Desertified Grasslands: their Biology and Management’ (ed. Chapman GP) pp 181-212. Academic Press: London
Ku MSB, Wu JR, Dai ZY et al. (1991) Photosynthetic and photorespiratory characteristics of Flaveria species. Plant Physiol 96: 518-528
Pinto H, Tissue DT, Ghannoum O (2011) Panicum milioides (C3-C4) does not have improved water or nitrogen economies relative to C3 and C4 congeners exposed to industrial-age climate change. J Exp Bot 62: 3223-3234
Portis AR Jr, Salvucci ME (2002). The discovery of Rubisco activase – yet another story of serendipity. Photosyn Res 73: 257–264
Portis AR Jr, Li C, Wang D, Salvucci ME (2008) Regulation of Rubisco activase and its interaction with Rubisco. J Exp Bot 59: 1597-1604
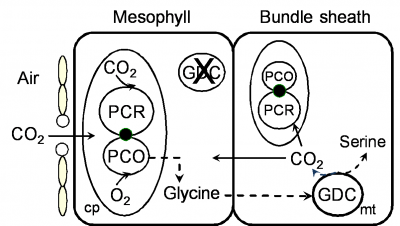
Rawsthorne S (1992) C3-C4 intermediate photosynthesis - Linking physiology to gene expression. Plant J 2: 267-274
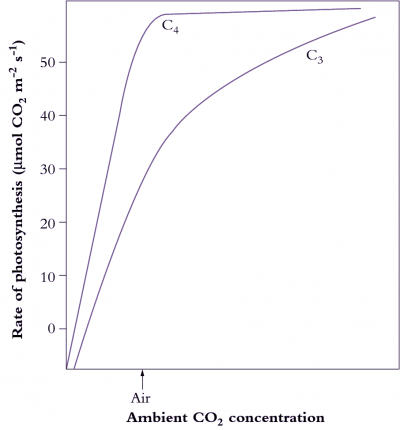
Sage RF (2004). The evolution of C4 photosynthesis. New Phytol 161: 341-370
Sage RF, Christin PA, Edwards EJ (2011) The C4 plant lineages of planet Earth. J Exp Bot 62, 3155-3169
Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53: 449-475
Vogan PJ, Sage RF (2011) Water-use efficiency and nitrogen-use efficiency of C3-C4 intermediate species of Flaveria. Plant Cell Environ 34: 1415–1430.
von Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosyn Res 77: 191–207
Voznesenskaya EV, Franceschi VR, Kiirats O et al. (2002) Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera. Plant J 31: 649–662
Voznesenskaya EV, Edwards GE, Kiirats O et al. (2003) Development of biochemical specialization and organelle partitioning in the single celled C4 system in leaves of Borszczowia aralocaspica. Amer J Bot 90: 1669-1680
8b44.jpg?itok=xCTYBDN2)