Ole Pedersen, University of Copenhagen, Denmark
Development of microelectrodes robust enough for use in field conditions has enabled in situ measurements of O2 dynamics in submerged plants (Figure 1). Eco-physiological research on submerged plants has been a challenge; infrared gas analyzers do not work under water! Field studies (in situ) have revealed how fluctuating light, diurnal changes in temperature and water column O2 concentrations all influence internal aeration of submerged aquatic plants. In the case of seagrasses, application of sulphide microelectrodes has also added to the growing evidence of sulphide poisoning as a likely cause of extensive die-backs.
CS_18.4-1.jpg

Figure 1. Diver operating O2 and H2S microelectrodes (left) in a Thallasia seagrass meadow in the Caribbean. Development of in situ equipment has enabled measurements of internal aeration under challenging field conditions in aquatic systems (right). (Photographs courtesy of Malene Hedegård Petersen (left) and Ole Pedersen (right).
CS_18.4-2.png
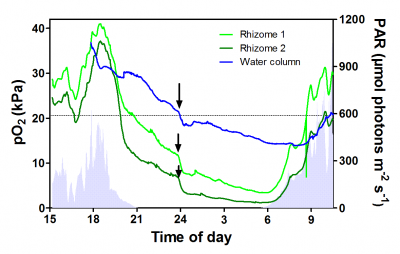
Figure 2. Oxygen dynamics in the rhizome of the seagrass Zostera marina during a diurnal cycle. O2 in the rhizome closely follows incoming light during daytime, whereas during the night internal aeration relies on supply of O2 from the water column. The dependence on water column pO2 during the night for internal aeration becomes particularly clear when the tide carries water across the seagrass meadow with lower pO2 as the internal pO2 of the rhizomes immediately declines (see arrows). During the day, it is the other way around as fluctuations in incoming light is immediately reflected in rhizome pO2 as daytime rhizome pO2 follows underwater photosynthesis. Data from Greve et al. (2003).
Seagrasses are flowering plants with roots, rhizomes, sometimes stems, and almost always strap shaped leaves to reduce pressure drag and thus the uprooting forces created by wave action. Seagrasses are key marine ecosystem engineers and habitat for various marine animals, but seagrasses are under world-wide threat from human activities (eutrophication, dredging and other physical disturbances). Eutrophication impacts directly on seagrasses by decreasing the available light (stimulates growth of epiphytes and planktonic algae) but also indirectly by stimulating the decomposition of organic matter in the sediment (mineralization of algae and seagrass litter is often limited by N and P) and thus increases the O2 demand of the sediment. In marine sediments, sulphate reduction by microorganisms can be substantial under anoxic conditions, producing sulphide, a potent phytotoxin with toxicity and mode of action similar to that of cyanide. Sulphide exists in three different chemical forms in water (H2S, HS- and S2-) with the gaseous H2S dominating at low pH and S2- at high pH. Gaseous H2S can enter the root and rhizome aerenchyma and move via diffusion to other parts of the seagrass, such as to leaf meristems that are relatively sensitive to sulphide. The resulting sulphide poisoning is a major cause of the worldwide die back of seagrasses observed in temperate as well as in tropical seagrass meadows.
Mechanistic field studies employing in situ microelectrodes have improved our understanding of internal aeration and sulphide intrusion in natural seagrass meadows. During the day, internal aeration of roots and rhizomes relies on O2 production in underwater photosynthesis, as does radial O2 loss (ROL) from roots to the sediments. There is a strong relationship between incoming light and O2 partial pressure in roots (Figure 2). Clouds immediately lead to a decline in root O2, whereas during periods of high sunlight root O2 was highest. During the night time, however, internal aeration relies on a steady flux of O2 from the water and into the leaves, and via the aerenchyma, further down into the roots and rhizomes.
CS_18.4-3.png
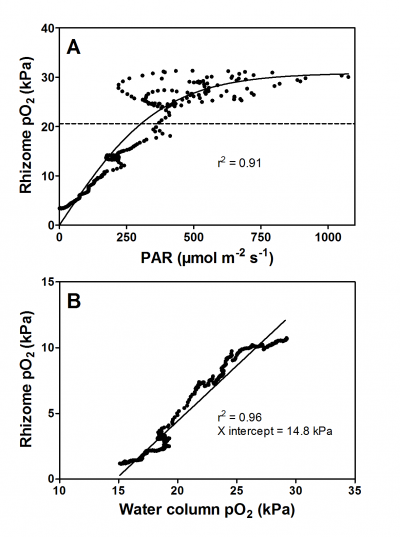
Figure 3. Oxygen dynamics in the rhizome of the seagrass Zostera marina during the day (A) and during the night (B). During the day, underwater photosynthesis produces O2 in the leaves and rhizome pO2 is thus a function of incoming light as O2 easily diffuses from the chloroplast and into the root via the porous tissues. In contrast, during the night, the water column is the only source of O2 for the plant and O2 diffuses from the water and into the leaves and further down into the belowground tissues. Consequently, rhizome pO2 is strongly correlated to water column pO2 during darkness. Re-analyzed data from Greve et al. (2003).
CS_18.4-4.jpg

Figure 4. Lobelia dortmanna and other isoetids in a Norwegian oligotrophic lobelia lake. The water of such lakes contains only little dissolved CO2 but L. dortmanna can take up CO2 from the sediment where the concentration is typically 100‑fold higher. As a consequence, L. dortmanna has no barrier to radial O2 loss (ROL) in its roots and almost all O2 produced in underwater photosynthesis is lost to the sediment via the roots. (Photograph courtesy of Ole Pedersen).
Critically low water column O2 can occur during nights in areas with still, warm waters, resulting from net system respiration faster than inwards movement of O2 into the stagnant waters. Under such night time conditions, roots can experience anoxia. Cessation of ROL to the sediments means there is no longer chemical oxidation of H2S to SO42- in the rhizosphere, so that gaseous H2S enters the aerenchyma and spreads via gas phase diffusion to all parts of the seagrass. The very metabolically active leaf meristems are thought to be particularly sensitive to sulphide poisoning. It is thought provoking that during most sudden die backs of seagrasses, the shoots are found drifting in the water with apparently healthy leaves but detached from the vertical stem exactly where the basal leaf meristems is located.
In contrast to coastal marine habitats of seagrasses, lobelia lakes are highly transparent oligotrophic, low alkaline lakes of the northern hemisphere. The vegetation consist of several evergreen species that are morphologically strikingly similar: short stiff leaves arranged in a rosette and with unbranched roots that can make up more than 50% of the biomass. As a whole, the type of vegetation is referred to as isoetids from the genus Isoetes that occurs in most lobelia lakes. Although not present in all lobelia lakes, Lobelia dortmanna is key species (Figure 4). Isoetids take up CO2 from the sediment via the roots and some are even CAM plants, although conservation of water is probably the least of all concerns for these plants. CO2 concentrations are highest at night, so CAM enables storage of malate for subsequent decarboxylation providing CO2 for photosynthesis the next day.
Many sandy sediments in shallow lobelia lakes are permanently oxic. Oxic sediments are a consequence of inherently low mineralization rates as the oligotrophic conditions lead to very low production of organic matter that is subsequently decomposed in the sediment. Nevertheless, the CO2 concentration in these sediments can be 100-fold higher than in the water above and L. dortmanna, along with the other isoetids, tap into this rich source of CO2 with their large root systems. The large gradient in partial pressure of CO2 between sendiment and photosynthetic leaves drives a flux of CO2 from the sediment, into the root aerenchyma (radial CO2 uptake) and then upwards into the porous leaves. Interestingly, the leaves are covered with a relatively thick cuticle to prevent loss of CO2 to the surrounding water, and as a result up to 100% of the O2 produced in underwater photosynthesis is lost via ROL from the roots (Figure 5).
CS_18.4-5.png
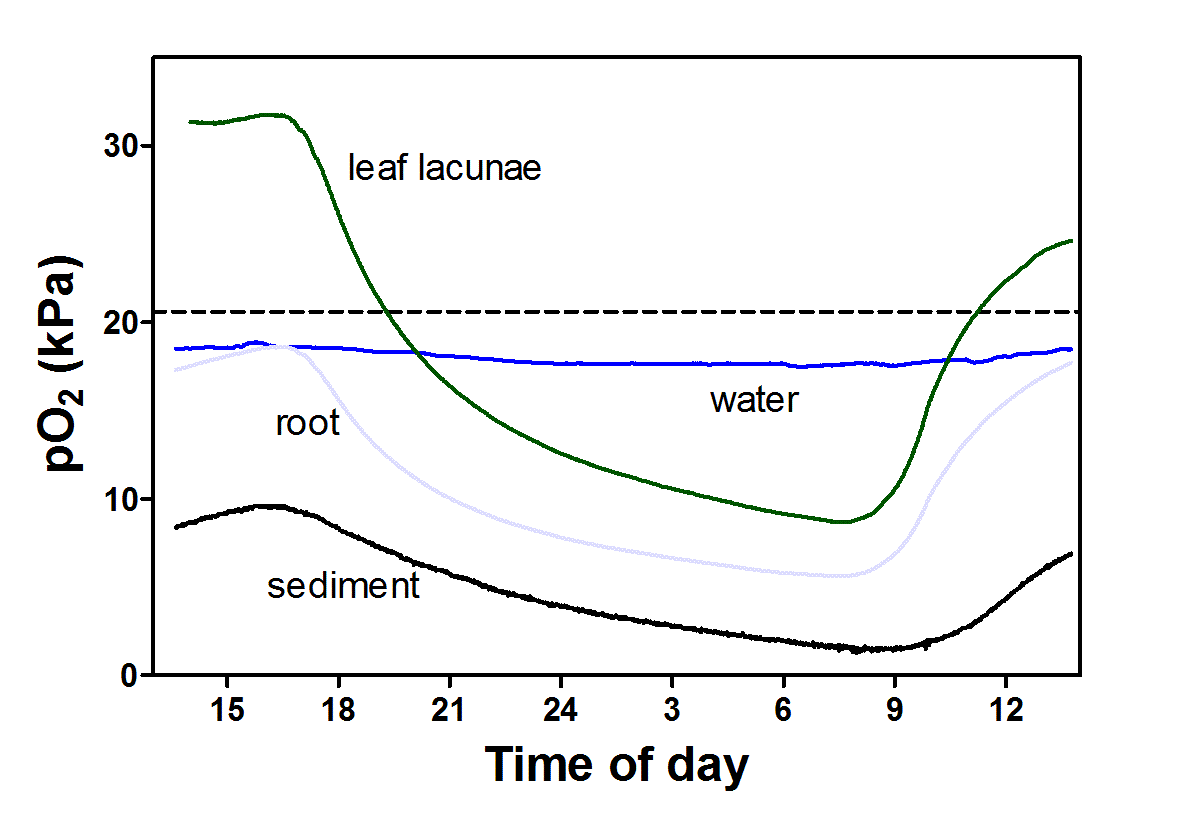
Figure 5. Oxygen dynamics in leaves and roots of Lobelia dortmanna and the surrounding water column and sediment in Lake Värsjö, Sweden. In light, underwater photosynthesis drives leaf pO2 up above 30 kPa and the steep gradient to roots results in a substantial O2 flux into roots. The roots have no barrier to ROL and thus the majority of O2 produced in photosynthesis is lost to the sediment resulting in large diurnal fluctuation in sediment pO2; in fact, the sediment remains permanently oxic. Data modified from Sand-Jensen et al. 2005.
In conclusion, the isoetids tested so far do not form a barrier to ROL in their roots and isoetids are thus restricted to sediments with very low O2 demand; any root barrier would also restrict CO2 uptake. In contrast, the few species of seagrasses studied all show a strong barrier to ROL and in the marine H2S rich environment the barrier would also reduce the inward flux of gaseous H2S.
References
Greve TM, Borum J, Pedersen O (2003) Meristematic oxygen variability in eelgrass (Zostera marina). Limnol Oceanogr 48: 210-216
Sand-Jensen K, Pedersen O, Binzer T, Borum J (2005) Contrasting oxygen dynamics in the freshwater isoetid Lobelia dortmanna and the marine seagrass Zostera marina. Ann Bot 96: 613-623
Pedersen O, Colmer TD, Sand-Jensen K (2013) Underwater photosynthesis of submerged plants – recent advances and methods. Front Plant Sci 4 DOI: 10.3389/fpls.2013.0014
