A.V. Spokevicius and G. Bossinger, School of Ecosystem and Forest Sciences, University of Melbourne
Trees are often big and difficult to work with. They have long generation times and many of the developmentally and commercially interesting characteristics, such as mature wood traits, are not observed until a tree is many years old. As a consequence, many aspects of the wood formation process remain elusive, such as the molecular control of wood formation and wood chemistry, which are commercially relevant. Much of our current knowledge about plant function and development has derived from the study of mutant and transgenic plants. In the absence of well-defined mutants, the functional analysis of many xylogenesis-related genes (responsible for wood formation) in forest tree species largely relies on transgenic approaches (Spokevicius et al., 2007b).
Many wood traits are extremely variable: most are influenced by programmed development (e.g. maturation of sapwood) and a range of environmental factors such as seasonal conditions which are expressed in wood growth rings. Such trait differences exist not only between trees but also within individual tree stems where they occur along the length (top to bottom) as well as across the woody stem. Identifying subtle changes in a wood phenotype by experimentally altering gene expression is therefore difficult unless a very high number of individuals is tested, a problem which is enhanced by tree size and the age of trait manifestation.
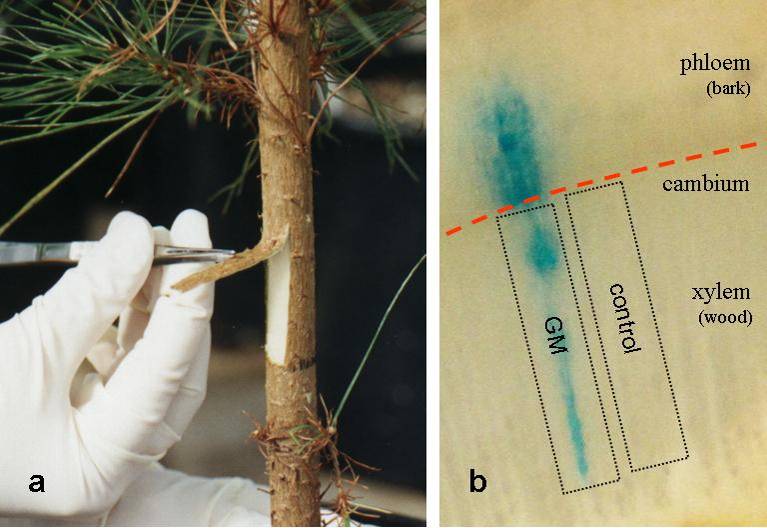
One method that has been successfully used to overcome many of these problems is an approach called Induced Somatic Sector Analysis (ISSA). In this method the bark is peeled from a small area of the trunk and an upward bark flap is created to expose the vascular cambium (Figure 1a). Dozens of flaps can be created on a single stem.
A suspension of the soil bacterium Agrobacterium tumefaciens, the ‘workhorse’ for plant genetic manipulation studies, containing the gene of interest is then applied to the exposed cambium facilitating genetic transformation and leading either to over-expression or down-regulation of the target gene in individual cambial cells. The bark flap is returned back into place and, following an initial wound response, further growth of successfully transformed cambial initials will lead to the formation of somatic tissue sectors (from within the existing tissue). These sectors include both newly formed wood and bark which are genetically distinct from surrounding untransformed tissue (seen as a blue sector caused by the GUS construct in Figure 1b). Morphological and biochemical characteristics can thus be compared between individual sectors and directly neighbouring control tissue after only a relatively short period of time (2-4 weeks) and without concerns about variability that might have been caused by genetic background or diversity due to treatment or other environmental conditions.
The use of ISSA has been instrumental for example in determining the role of the \(\beta\)-tubulin gene in secondary fibre cell wall formation during wood formation in eucalypts. Secondary fibre cells are complex structures that collectively give rise to wood. Their walls largely consist of helically aligned cellulose microfibrils that are embedded in a matrix of lignin and hemicellulose and arranged in three distinct secondary cell wall layers (S1, S2 and S3) lying inside the primary wall of each fibre cell. The S2 layer is the dominant cell wall layer in this arrangement and the angle at which S2 cellulose microfibrils are aligned relative to the long axis of the cell is called the microfibril angle (MFA). MFA is an important ecological and economical trait as it determines the strength and flexibility of individual fibre cells and hence the woody tissue that is composed of these cells. A tree’s resistance to gales and structural utility as timber depend upon MFA. So, by creating and analysing the genetically modified wood sectors, scientists were able to demonstrate that the \(\beta\)-tubulin gene was involved in cellulose microfibril orientation in the S2 layer of secondary fibre cell walls. These experiments provided first clues about the genes and subsequent molecular events that enable trees to stay aloft. Biotechnology based on these genes and manipulation of MFA could thus be targeted by molecular editing.
Substantial tree bioinformatic resources such as the sequencing of complete poplar and eucalypt genomes are now providing unprecedented scope for gene manipulation, and unravelling of the process of wood formation. However, the elucidation of specific gene function during wood formation is likely to remain a task for ISSA and other similar transgenic approaches for the foreseeable future.
References
Spokevicius AV, Southerton S, MacMillan CP et al. (2007a) \(\beta\)-tubulin affects cellulose microfibril orientation in plant secondary fibre cell walls. Plant J 51: 717-726
Spokevicius AV, Tibbits J, Bossinger G (2007b) Whole plant and plant part transgenic approaches in the study of wood formation – benefits and limitations. Transgenic Plant J 1: 49-59
Van Beveren KS, Spokevicius AV, Tibbits J et al. (2006) Transformation of cambial tissue in vivo provides an efficient means for induced somatic sector analysis and gene testing in stems of woody plant species. Funct Plant Biol 33: 629-638
Xu T, Ma T, Hu Q, Liu J (2015) An integrated database of wood-formation related genes in plants. Scient Rep 5: 11422