B. E. S. Gunning, Research School of Biology, Australian National University
Cell walls determine most of the fundamental features of the Plant Kingdom.
More than one billion years ago certain key evolutionary events set the cells that were to become the progenitors of plants apart from the other primordial organisms. What were these defining attributes and what part did they play in founding the Plant Kingdom?
Some would say that photosynthesis was the key to plant evolution. It arose first in prokaryotes and later passed to eukaryotes. Certainly it was essential, but was it alone sufficient to trigger evolution towards the Plant Kingdom? The theme of this case study is that the full potential of photosynthesis could not be realised by the progenitors of plants until they had evolved a suitable cellular environment, of which a vital component is a cell wall. Photosynthesis still occurs in unwalled, evolutionary dead-ends like Euglena, reinforcing the view that a truly seminal cellular state was only achieved when photo-synthesis in a eukaryotic cell was combined with a cell wall. Consider now how cell walls confer unique features on plant cell organisation and function, and how they underpin the entire lifestyle and marvellous diversity of plants.
Cell walls: strength through osmotic regulation
Why did the first eukaryotic, photosynthetic, walled cells have such distinctive evolutionary potential? Probably all life was aquatic at the time and regulating water and solute balance (osmotic regulation) was critical for survival. The earliest cells were almost certainly in osmotic balance with the fluid in which they lived. However, as cell metabolism became more complex, internal solute concentrations rose. If solute concentrations in the surrounding medium dropped (for example, if it rained), water would be taken up by cells to equalise the osmotic pressure and they would swell. Cells which do not have walls, like Amoeba, control swelling by expelling water, otherwise they would burst (Figure 1). A cell wall containing strong but flexible micro-fibrils offered the progenitors of the Plant Kingdom an alternative solution. Hydrostatic pressure created by water flow into cells is opposed by the mechanical strength of cell walls generating wall pressure. In living cells, this neatly balanced cell turgidity is maintained by control of osmotic processes, mechanisms for turgor sensing and organisation of cell wall composition.
7.1-CS-Fig-7.1.jpg
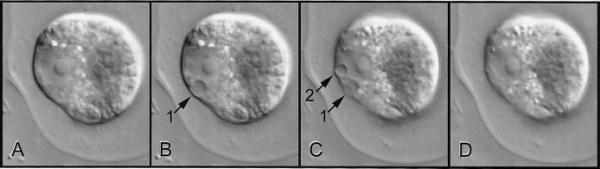
Figure 1 Contractile vacuoles filling and emptying in a Chlamydomonas cell. Four pictures were taken about two minutes apart to illustrate the filling and emptying cycle of two contractile vacuoles in the cell. A, The two contractile vacuoles are empty and invisible at this magnification. B, One vacuole has filled (arrow). C, This vacuole is emptying and the second vacuole is filling. D, Both vacuoles have emptied again. Part of the boundary of the gelatinous envelope that surrounds the cell is visible in each picture (Micrographs courtesy B.E.S. Gunning)
Most plants adjust the osmotic properties of their cells so that they can live in a turgid state regardless of the water potential in their environment. This brings great advantages for plants. The balance of forces in a turgid cell generates more than just water balance. It confers rigidity and mechanical strength, as witnessed by comparing a wilted leaf with a turgid leaf. Another way to gain strength is, of course, to synthesise thick cell walls. However, organs composed mainly of thin-walled cells, like leaves, can support themselves if they are turgid. They therefore do not need to synthesise the large amount of wall material that would be required if strength relied solely on wall rigidity. This is especially important in growing regions of plants where cells must enlarge. Primary cell walls also confer enough strength on tissues for them to hold their shape and form — for example, enough to let a root tip penetrate through soil. In general, as cells mature the plastic properties of their walls give way to increasing rigidity.
Here, then, at the dawn of the Plant Kingdom, was a new form of osmotic regulation with many inherent evolutionary possibilities. It proved to be a springboard for the appearance of other novel features of plant cell structure and function.
Cell walls, vacuoles and cytoskeleton: partners in production of large cells
Many distinguishing features of plant cells relate to cell walls. Vacuoles, for example, are found in the vast majority of plant cells, but seldom in animals. They probably evolved from an original digestive (lysosomal) compartment, indeed they still have some digestive roles in plant cells. Now one of their main roles is to store osmotically active solutes, thus partnering cell walls in maintaining turgor. In so doing they have a huge impact on the architecture and size of plants. Their presence permits economical production of large cells in which a small amount of biosynthetically expensive cytoplasm is distributed as a thin film over a large surface area between the wall and vacuole(s). Large, turgid, vacuolate, walled cells are in turn economical building blocks for increasing body size. In fact some 90% of all increase in volume during plant growth comes from an enlarging vacuolar compartment and concomitant stretching of cell walls. This process transforms small, densely cytoplasmic, meristematic cells into mature, vacuolate cells.
7.1-CS-Fig-7.2.jpg

Figure 2 Microfilaments of actin. In this elongating cell from a wheat root tip, strands of actin ramify through the cytoplasm, mostly running along the length of the cell. They are stained here with a fluorescent antibody and viewed by confocal microscopy. The cell nucleus is just visible, lying in the right-hand end of the cell. Actin protein polymerises into microfilaments, and these often aggregate into bundles such as those imaged here. Polarity of the actin molecules determines the direction of cytoplasmic streaming along the microfilaments (Micrograph courtesy B.E.S. Gunning)
Walls and vacuoles together give the opportunity to make big cells and hence big plants, but this potential can be realised only if an associated metabolic problem is overcome. Thousands of biochemical reactions are needed to support life. For them to proceed fast enough the interacting molecules must collide sufficiently frequently. Simple diffusion in the confined volume of small cells allows them to do this — one of the advantages of being small. Frequency of collisions drops off greatly if the colliding molecules have to diffuse over longer distances, or are present in dilute solutions, as might happen in cells that have taken advantage of walls and vacuoles to enlarge dramatically. One way in which this potential physical limitation on life processes is alleviated in present-day plant cells is that a cytoskeletal system stirs and mixes the cytoplasm. The process is visible in most large walled cells and is fascinating to watch. Like stirring reactants in a beaker, it helps to overcome diffusion barriers.
Actin and tubulin are ubiquitous components of the cyto-skeleton of plants and animals. Actin molecules are the units of ‘microfilaments’ which provide tracks for cytoplasmic streaming (Figure 2). To achieve streaming, actin acts in concert with myosin (another cytoskeletal component), proteins and ATP as an energy source for mixing. Molecules of tubulin are polymerised to make ‘microtubules’, which have multifarious roles in living cells (Figure 3). Special roles related to cell wall development are discussed below.
7.1-CS-Fig-7.3.jpg
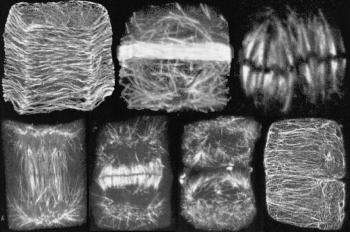
Figure 3 Microtubules have many roles during the cell division cycle. The following stages can be seen in cells from a wheat root tip stained with fluorescent-labelled antibody to the protein tubulin (from left to right): (i) pre-(or post-) division, when microtubules lie transverse to the long axis of a cortical cell, just under the plasma membrane. Microtubules govern congruent deposition of cellulose in the growing cell wall; (ii) the cell has become committed to divide and is establishing the future site and plane of division by laying down a dense band of microtubules (the ‘pre-prophase band’) that passes right around the cell; (iii) the metaphase stage of mitosis, with chromosomes lined up on the equator of the division figure, connected to poles of the mitotic spindle by bundles of microtubules that ultimately separate daughter chromosomes; (iv-v) early and later stages of development of the ‘phragmoplast’, an apparatus of microtubules and actin in which a new cell wall is initiated between the daughter nuclei; (vi) division almost complete, with just a few remnants of the phragmoplast visible and two daughter cells almost separated, although their cortical microtubules are not yet recognised; (vii) daughter cells have formed new arrays of cortical microtubules, similar to those of stage (i). (Based on Gunning and Steer 1996)
Cell walls have a unique biosynthetic apparatus linked to unique cell morphogenesis
Plant cell walls comprise two phases: microfibrils (mainly of cellulose, the world’s most abundant biopolymer) are embedded in a gel matrix of other polysaccharides and some very specialised proteins. Two distinct sets of biosynthetic apparatus generate the microfibrils and the matrix components, bringing further unique features to plant cell organisation.
7.1-CS-Fig-7.4.jpg
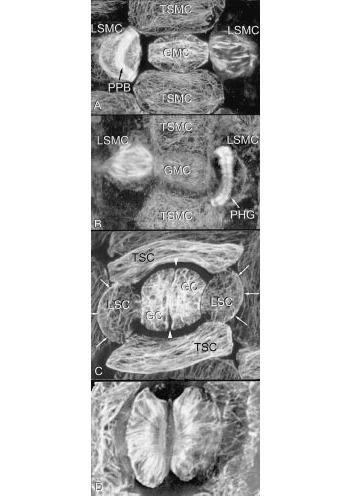
Figure 4 The basic types of microtubule array can vary greatly in specialised cells and tissues. Developing stomata show many complexities, including asymmetrical cell divisions and formation of cell walls with unusual microfibril reinforcement. Four stages of formation of Tradescantia stomata are shown here, using microtubule staining. In A, the central guard mother cell (GMC) is surrounded by terminal and lateral subsidiary mother cells (TSMC and LSMC); here the LSMC on the left has a curved pre-prophase band (PPB) which predicts the shape of the future wall of the subsidiary cell (arrows in C).The LSMC on the right is in mitosis. B, This shows a later stage, with the LSMC on the left in mitosis and that on the right with a curved phragmoplast (PHG), also predicting the shape and position of the future wall. C, Divisions are complete. Arrows show the walls that were formed successively under the influence of the pre—prophase band and phragmoplast. The guard mother cell seen in A and B has now divided longitudinally (between arrowheads) to form two guard cells (GC) in the stomata. All four subsidiary cells (two TSC and two LSC) have now been formed. During differentiation of guard cells, microtubules in the cell cortex radiate from the future pore (D). Cellulose microfibrils are deposited in this orientation, creating a cell wall that can respond to turgor changes in such a way that the stomatal pore can be opened and closed (Micrographs courtesy A. Cleary)
One biosynthetic apparatus consists of cellulose-producing enzyme complexes in the plasma membrane. They often work under the guidance of an array of microtubules that lies at the inner face of the plasma membrane and directs growing cellulose chains into specific orientations. This is a vital regulatory system because the strength of cell walls depends on the orientation of its microfibrils. The microtubule cytoskeleton lying beneath the plasma membrane is a tool by which cells control the local directional strength of their walls. Microtubule arrays indirectly determine the shape that a cell assumes when it is stretched by turgor (Figure 4). There is nothing like this combination of membrane-based synthesis and guidance by cytoskeletal microtubules in animal cells. In plants it is a major mechanism of cell shaping and lies at the heart of much of plant morphogenesis.
The second biosynthetic apparatus for wall production does have a counterpart in animals, but the flavour is different, thanks again to the wall itself. All eukaryotic cells have an elaborate system of membranes in which certain proteins are made, modified and secreted. Most of the proteins secreted by animal cells are glycoproteins, that is, proteins with carbo-hydrate side-chains attached to them. A special region of the membrane system, the Golgi apparatus, adds these side-chains. Plants also make glycoproteins, but the great bulk of their Golgi activity is given over to manufacturing cell wall matrix polysaccharides. This differing biosynthetic emphasis might account for differences in organisation of the Golgi apparatus in plants and animals. In animal cells, Golgi bodies are usually central, near the nucleus, whereas in plant cells they are widely dispersed in multifunctional ‘Golgi-stacks’ of membranes. After the wall matrix materials have been made, vesicles containing them are delivered from Golgi stacks to particular regions of the cell surface and thence to growing cell walls. Especially in large cells, this intracellular movement depends once again upon the actin-based cytoplasmic streaming system that evolved in walled cells.
7.1-CS-Fig-7.5.jpg
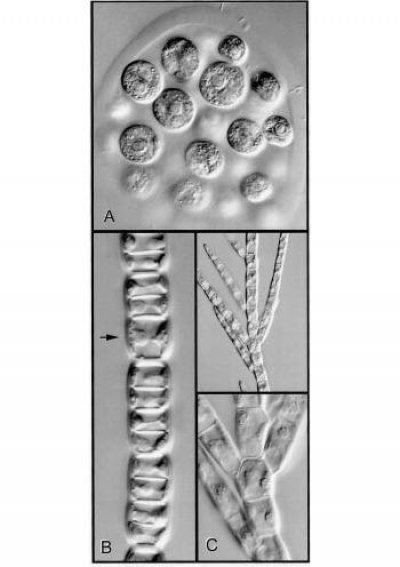
Figure 5 Cell walls, planes of cell division and the form of a plant body are illustrated in genera of green algae. A, Colony of Eudorina. The constituent cells are embedded in a gelatinous matrix. At the end of cell division daughter cells separate from one another. B, This shows what happens when division is always in one plane. Sharing of new cross-walls by daughter cells causes them to adhere to one another. The arrow indicates a cell that was about to divide. C, A vital new feature — the ability to change the plane of division generates branching systems of adherent cells (Stigeoclonium, low and high magnification views). (Micrographs courtesy B.E.S. Gunning)
Two elaborate cytoskeletal devices place new cell walls accurately (Figures 3 and 4). The first is a preparation for cell division. The cytoskeleton of the parent cell establishes the site and plane of division even before the nucleus undergoes mitosis. This cytoskeletal apparatus (pre-prophase band) is not found in present-day algae (although many algae can still control the plane of division in their cells) and may have arisen after the algal stage of plant evolution. A second cytoskeletal structure initiates the actual fabrication of new cell walls. It is initiated between daughter nuclei and grows outwards to join the parental walls at a predetermined site. This apparatus, termed a phragmoplast, did evolve in advanced algae and occurs in the ancestors of higher plants. Neither of these cytoskeletal devices for establishing and implementing precise sites and planes of cell division occurs in animals.
7.1-CS-Fig-7.6.jpg
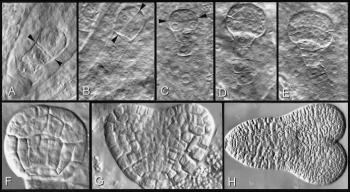
Figure 6 Cell walls, planes of cell division, and form of the plant body illustrated through embryogenesis in a higher plant. Embryo formation in Arabidopsis provides an example of highly regulated planes of cell division during formation of a specifically shaped plant body. Arrowheads in A to C show successive planes of division in very young pro-embyos. Subsequent divisions (D-H) build up a heart-shaped embryo with surface and inner walls and embryonic root and cotyledons. D, The complete suspensor filament as well as the globular pro-embryo. In A to E the embryos are embedded in endosperm tissue in the embryo sac; in F to H they have been isolated from their embryo sacs. (Based on Gunning and Steer 1996)
The cell wall: constraints and opportunities in nutrition
Cell wall properties have implications for plant nutrition. The close-knit fabric of cell walls sieves out all but very small nutrient molecules. This rules out a feeding mechanism that was probably common in early life forms — engulfing particles of food in loops of plasma membrane and internalising them for digestion. The first walled cells had to adapt their nutritional habits leading to at least two evolutionary outcomes. Present-day fungi subsist on external food sources by secreting enzymes that digest macromolecules sufficiently to allow the products to pass through cell walls. Roots of higher plants also secrete extracellular enzymes such as phosphatases which liberate inorganic phosphate. Higher plants also entered into an intra-cellular symbiosis with photosynthetic organisms, which then served as internal sources of organic carbon compounds. This led to green plants, whose present-day chloroplasts are held to be much-modified descendants of originally free-living photo-autotrophs. Symbiotic association between walled hosts and photosynthetic partners laid the foundations for a magnificent diversity of plant life, mentioned at the start of this case study.
Cell walls circumscribe pathways of transport within plants
Another adaptation of cell walls allowed early colonists of the land to develop division of labour between roots and shoots and rise to the airy heights of fields and forests. External cuticle layers, which reduce loss of water to the atmosphere, let cells of aerial parts survive provided that water could be delivered from plant organs in contact with external sources, mostly roots. Numerous other adaptations of wall structure occur, some related to mechanical strength or protection, and many to transport of water and nutrients.
7.1-CS-Fig-7.7.jpg
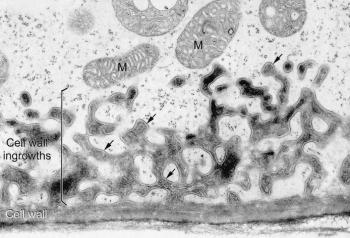
Figure 7 Transfer cell wall. Elaboration of a cell wall into projections that are lined by plasma membrane (arrows), thus providing an enhanced surface area for exchange of many different types of solutes. Transfer cells develop in various plant tissues involved in transport. Mitochondria (M) are usually found in the vicinity of the wall labyrinths (A. Browning and B.E.S. Gunning, freeze-substituted transfer cell in the haustorium of a Funaria sporophyte, based on Gunning and Steer 1996)
The molecular construction and small pore sizes of cell walls limit the size of molecules that can be transported around plant bodies. One pathway of transport consists of the interconnected lattice of cell walls themselves — the ‘apoplasm’. Impregnation of the wall matrix with hydrophobic substances creates apoplasmic barriers in some strategic locations; in other locations the apoplasm is open and permeable. In ‘transfer cells’, fingers of wall protrude into the cytoplasm and provide an unusually large surface area for transport across the adjacent plasma membrane (Figure 7). Many sites of intensive absorption or secretion possess this wall adaptation.
From the very early evolution of multicellular plants, fine cylindrical extensions of cytoplasm — plasmodesmata — have pierced the wall between adjacent cells. By passing through cell walls and the middle lamella, plasmodesmata form a transcellular commune of living cell contents known as the ‘symplasm’. Through this important cell to cell transport pathway, bounded by a continuous plasma membrane system, tissues evade some of the transport constraints imposed by cell walls.
Division of labour into roots, stems, leaves, meristems and other organs depends upon mass transport of metabolic products. Because solutes transported around plants must sometimes traverse cell walls, there can be no equivalent of the blood-stream of animals, which delivers macromolecules in a mass flow. Only small, wall-permeable molecules (e.g. sucrose and amino acids) are suited for mass transport in plants. In turn, the need to import and export these small molecules determines the nature of many biochemical pathways and physiological systems in plants.
Cell walls: a sensory and signalling system
7.1-CS-Fig-7.8.jpg
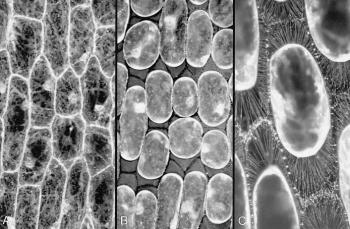
Figure 8 Connections between the plasma membrane and cell wall. A, Some cells in an onion bulb scale leaf epidermis, stained with the fluorescent dye DIOC(6) and viewed by confocal microscopy. Cytoplasm is seen as pale strands at the cell surface, traversing the large vacuole and often passing to the nucleus. Cell boundaries are bright because the surface cytoplasm (especially endoplasmic reticulum) is intensely fluorescent. B, Precisely the same field of view after plasmolysis in 0.6 M sucrose. The cell walls are now visible as dark lines between the shrunken protoplasts, which still show brightly fluorescent surfaces. C, A reconstruction of many planes of focus at a higher magnification to show some of the hundreds of stretched strands of plasma membrane that connect the protoplasts to the cell wall. These strands form because molecules in the plasma membrane (and peripheral cytoplasm) remain tethered to the wall during plasmolysis. The plasma membrane therefore becomes pulled out into very fine strands when the protoplasts shrink. (A and B are based on Gunning and Steer 1996; C, micrograph courtesy B.E.S. Gunning)
Plasma membranes and cell walls meet at a very special interface: a living cell abuts a non-living but chemically active external covering. Here the cell perceives much about the outside world. Innumerable connections between the inner face of the wall and the outer face of the plasma membrane are revealed by plasmolysis (Figure 8). These fine strands are indicative of molecules that link walls to plasma membranes. In some places links extend even further, connecting the wall through the plasma membrane to strands of endoplasmic reticulum and perhaps to elements of the cytoskeleton. These strands are ideally located to transmit physical signals arising from mechanical disturbance at the wall–membrane interface. Wall pressure in expanding, turgid cells might be communicated via these strands to activate membrane processes such as mechanosensitive channels.
Classical plant biology points to roles for a linked wall–membrane sensory apparatus. Charles Darwin showed that bean roots are 100 times more sensitive to touch than human touch receptors. Stimuli that he himself could not perceive alter root growth patterns. Plants respond dramatically to touch and to stretching and compression of cell surfaces. ‘Wind pruning’ of trees is a familiar example of a large-scale effect. Specialised touch receptors occur in tendrils and insectivorous plants. They also trigger mechanical pollination mechanisms. All such stimuli are perceived at the outer face of a cell wall, whence signals pass to and are transduced in the underlying cytoplasm.
Another class of wall-mediated sensing deals with chemical rather than physical stimuli. Plant cells detect certain short chains of sugar residues (oligosaccharides), derived from enzymatic hydrolysis of cell wall polysaccharides, with extraordinary sensitivity and specificity. This gives plants early warning of attack by pathogens, which normally have to digest their way through the cell wall as they begin their infection, liberating oligosaccharide signal molecules as they penetrate. Some of the plant’s own hormonal signalling system probably also uses oligosaccharides, independent of pathogen attack. In other words, the wall contains messages built into its molecular construction, ready to trigger growth or defence responses when released. More than most other phenomena, this illustrates the subtlety with which the cell wall is integrated into the life of plants.
Cell walls: chemical and functional diversity
Many constituent molecules give rise to hundreds of different wall polymers with diverse functions. Classical staining reactions to identify wall components are giving way to new approaches to wall function. Molecular probes such as antibodies and separation techniques give deeper insights into the great diversity and specificity of wall composition. Polysaccharides, for example, can be extraordinarily complex with wide-ranging variation in constituent sugar units, branching patterns, sequences and substituents. They can thus be extraordinarily specific in signalling and recognition systems.
Part of the chemical diversity of cell walls is related to functional diversity of cell walls in varied roles such as skeletal support, waterproofing, deterring herbivores, sustaining tension in the transpiration stream, protection of specialised cells like pollen grains from desiccation, and so on. Increasingly, however, very subtle chemical modifications of walls are viewed as ways in which cells can recognise each other and their positions in tissues and organs. There is now an appreciation of the role of chemical signalling as a guide to cell fate in plants, analogous to cell signalling systems in animals.
Cell walls: consequences of a sedentary lifestyle
Cell walls impose a sedentary lifestyle on plants. With few exceptions, mobility of plants is limited to local movements of plant parts, explorations of the environment by growth of individuals and colonisation by reproductive units. Inevitable outcomes of being rooted to the spot include intense neighbourhood competition below ground for water and nutrients and above ground for light, adaptations to varied environments, subtle environmental sensing mechanisms, amazingly diverse chemical, physical and sacrificial defence strategies, breeding systems that employ mobile organisms to disperse propagules, and a type of cell wall that protects the only really mobile cell category, pollen grains, during their aerial journey. Events at all levels in plant biology are influenced by this sedentary lifestyle. That the habit comes from the evolutionary decision to regulate osmotic properties by means of a cell wall is not always explicit, but the underlying fact is there!
Cell walls: a focus of current research
Strictly, cell walls are not alive but to dismiss them as an inert and uninteresting box around cells could not be further from the truth. Cell walls are the major determinant of plant form and function, whether viewed at the level of individual cells, whole-plant physiology or characteristics of the Plant Kingdom.
Not surprisingly, cell walls are one of the main foci of modern research in plant science. Their chemical complexity demands new techniques for separation, purification and analysis of their constituents, as well as studies of how the molecules interact and cross-link in the intact wall. Such knowledge is needed to understand how cells grow and recognise each other. Advanced computing is being added to biochemistry and biophysics in efforts to unravel the ‘micro-engineering’ properties of walls, necessary for looking at larger aspects of growth, for instance in shoot meristems where sheets of cells stretch, deform and grow out into leaf primordia. Increasingly the powerful methods of molecular genetics are being brought to bear. Already many mutants have been isolated with specific deficiencies in wall components, leading in turn to abnormal behaviours in growth, development and physiology. The research spectrum stretches from basic science to practical applications, the latter stemming from uses of cell walls in fibre, paper, fabrication, fuel and chemical industries. As usual, for the practical applications to prosper, plant scientists must learn much more about the basic biology — we must ‘first know the nature of things’.
Reference
Gunning BES, Steer MW (1996). Plant Cell Biology. Jones and Bartlett, Melbourne.
