Plants, fungi and bacteria produce a host of volatile compounds. Some attract or repel animals, some create powerful emotions in humans and some induce morphological and metabolic changes in adjacent plant tissues. Of all these emanations only ethylene is recognised as a natural gaseous plant hormone.
Ethylene has been used unintentionally to manipulate crops such as fig as far back as the third century BC. The sycamore fig originated in eastern central Africa, where it was naturally pollinated by a small wasp that makes its home inside the fruit. When the sycamore fig was taken into the eastern Mediterranean countries, including Egypt, pollinating wasps were left behind. Nevertheless, young fruit which were mechanically injured set parthenocarpically and ripened without seed! A 1633 herbal noted that ‘It bringeth forth fruit oftener if it be scraped with an iron knife, or other like instrument’. The fruit is ‘like in juice and taste to the wilde fig, but sweeter, and without any grains or seeds within’. We now know that wounding young fruit would have stimulated ethylene production and this gas induced those figs to grow and develop parthenocarpically. David Blanpied summed up this piece of history by recasting Amos 7:14 (OT), ‘I was no prophet, neither was I a prophet’s son; but I was an herdsman, a gatherer of sycamore fruit’, as ‘I was an herdsman and an activator of ACC synthase in sycamore figs’ (Blanpied 1985).
Blanpied’s quotation nicely sums up the history of ethylene as a plant hormone because it takes us from simple fruit behaviour to underlying biochemistry. Once the presence of ethylene in plant emanations was proved chemically, a lively debate followed as to whether a gas could really be defined as a hormone. There were two major developments that resolved this issue. First was the invention of gas chromatography which soon enabled measurement of ethylene at concentrations that were physiologically meaningful and in small gas volumes. Second, a non-volatile plant product, 1-aminocyclopropane-1-carboxylic acid, was found to be the immediate precursor of ethylene (Adams and Yang 1979). Any lingering doubts that ethylene was a plant hormone have now been completely erased by application of molecular methods.
This story of ethylene mixes applications of plant physiology with human intuition, and is conveniently related to three eras that represent technical evolution in this area of plant science, namely, pre-1935 (an age of mystery), 1935–1979 (an age of enlightenment) and post-1979 (an age of opportunity).
An age of mystery
In 1858, Fahnestock in the USA observed that illuminating gas caused plant senescence and leaf abscission, and Girardin (1864) in France subsequently showed that ethylene was a component of illuminating gas. Many suspected that such plant responses were due to ethylene, but it took a Russian student, Neljubov (1879–1926), to establish that ethylene is a biologically active compound. As a young man, he observed that pea seedlings germinated in the dark grew in a horizontal direction when exposed to laboratory air containing burnt gas. He showed that the plants resumed normal growth when the air was first passed over heated CuO to oxidise hydrocarbon gases. This growth response was used as a bioassay for the next 50 years. We now know that these pea seedling responses are induced by as little as 0.06 µL L–1 ethylene.
Many publications from around 1910 indicated that ethylene was produced by ripening fruit such as pears and apples. By 1923, Denny (US Department of Agriculture) had patented ethylene for ripening bananas, tomatoes and pears, removing astringency from persimmons and loosening walnut husks. Finally (1934) Gane in Britain produced conclusive proof that ethylene is a natural product of plants, and to obtain enough ethylene for his tests he collected gases from about 28 kg of apples. He extended this proof to other fruits a year later.
An age of enlightenment
Following Gane’s confirmation that ethylene generation was common in fruits, research interests broadened beyond this simple ethylene–fruit connection. By 1940 the postharvest pioneer Jacob Biale (University of California, Los Angeles) showed that green citrus mould (Penicillium digitatum) also produced ethylene, thereby extending ethylene physiology to plant–fungus interactions. Hormonal interrelations entered this picture when the synthetic auxin 2,4-D was later shown to stimulate ethylene production by plants. Ethylene was by now acknowledged as instrumental in fruit ripening, but a nagging question remained as to whether ethylene was a true ripening hormone or merely a by-product of ripening events. I entered the field at this stage, and to resolve this issue of hormone status we needed to establish whether ethylene production by fruits increased ahead of ripening. Progress in unravelling cause and effect would hinge on development of a sensitive assay for ethylene.
Strong indications of ethylene involvement in ripening came from experiments using cold mercuric perchlorate solutions to bind specifically ethylene rather than other gases, and thus trap a sufficient amount from ripening tomatoes to measure it manometrically. However, the much greater sensitivity of gas chromatography subsequently allowed demonstration via frequent monitoring that ethylene production actually precedes the onset of ripening in some fruit.
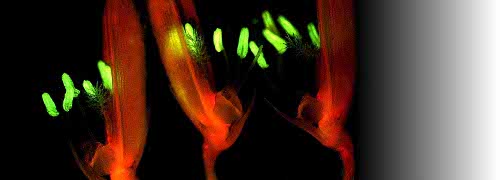
Scientifically, these were exciting times. As a PhD student at the University of California, Davis, I was a member of one of the first teams to use a gas chromatograph fitted with a flame ionisation detector to measure internal ethylene concentrations in a ripening fruit (Lyons et al. 1962). We showed conclusively that cantaloupe (rockmelon) was climacteric. Harvested fruit showed an increase in ethylene production with onset of a respiratory climacteric and ripening. Over the next 20 years an explosion of publications documented ethylene involvement in many plant responses. Burg and Burg (1960s) demonstrated that ethylene was essential for ripening as well as other developmental events in plants. Senescence is a case in point, and a clear ethylene response is shown in Figure 2 for Cymbidium flowers.
Fig 2_3.jpg

Figure 2. Ethylene generation influences postharvest behaviour of Cymbidium flowers. When the pollen cap is removed from the floral column either by insect pollination or by human mishandling, endogenous ethylene production is triggered in that flower (left side), bringing about anthocyanin synthesis, cupping of petals and swelling of column tissues within 3 d. Intact flowers (right side) remain fresh for three weeks. Scale bar = 1 cm. (Photograph courtesy R.L. Bieleski)
A further practical development from ethylene research dates from 1963 with synthesis of ‘Ethephon’ (2-chloroethyl-phosphonic acid) (also called ‘Ethrel’). This water-soluble compound is readily absorbed by plants, and breaks down to release ethylene above pH 4.6. Ethephon thus provides a convenient way of applying ethylene to plants under field conditions and it has been used to promote uniform maturation of processing tomatoes as an aid to mechanical harvesting.
Three broad research themes in ethylene physiology were now underway: mode of action, inhibition of action and biosynthesis. However, a major problem confounding our best efforts in all three areas was the autocatalytic behaviour of ethylene. This gas stimulates its own production, so how do you distinguish between the external ethylene you have applied experimentally as a stimulus, and the endogenous ethylene which is produced as a response by the plant tissues? Confronted by this dilemma, we devised a neat trick based upon a closely related gas (McMurchie et al. 1972). Propylene is a three-carbon analogue of two-carbon ethylene, although about 100 times less active than ethylene, that can stimulate typical ethylene responses! Moreover, propylene is also easily distinguished from ethylene by gas chromatography. We now had an elegant tool for analysis of ethylene physiology.
We applied propylene to citrus fruit (non-climacteric) and to bananas (climacteric) to mimic an exogenous ethylene stimulus, and measured endogenous ethylene production directly. Citrus respiration was stimulated without any increase in ethylene production, whereas in banana both respiration and endogenous ethylene production were stimulated. These outcomes were consistent with our paradigm of ripening in climacteric versus non-climacteric fruit.
Once ethylene was widely acknowledged as a ripening hormone, there was a strong demand by industry for practical control methods in order to extend fruit storage life. Our original approach was to remove ethylene from fruit storage atmospheres by scrubbing with oxidising agents such as permanganate. Commercial absorbents containing permanganate are available but inconvenient to use because the absorbent has to be packaged to prevent contact with stored fruit. The search for other ways of avoiding or inhibiting ethylene action continued. By 1976, Beyer showed that silver ions are a potent inhibitor of ethylene action, and a new set of management options opened up immediately. Ag+ is readily bound by plant tissue but not easily translocated and is thus of limited application. However, the silver thiosulphate complex (STS) is negatively charged and can move readily through plant tissues. This observation had little practical value for fruits which are eaten, but has had wide use in slowing the ethylene-driven senescence of cut flowers. In 1979 Sisler introduced volatile unsaturated ring compounds as inhibitors of ethylene action, the most potent being norbornadiene. Sisler has subsequently developed 1-methylcyclopropene (1-MCP), a gaseous compound which is essentially an irreversible inhibitor and safe to use (Sisler and Serek 1997).
While ethylene was gaining wider application in postharvest physiology, research continued with unravelling the biosynthetic pathway. The first clue came when Lieberman and Mapson (1964) supplied the general precursor [14C]-methionine to ripening tissue and found 14C in the ethylene produced. Methionine had been noted as a possible precursor from the discovery that rhizobitoxin inhibits ethylene production. Rhizobitoxin inhibits pyridoxal phosphate-containing enzymes of the kind involved in methionine-utilising pathways. A commercial product (Retain™) containing aminoethoxyvinylglycine (AVG) is now used as a preharvest treatment to delay ripening of apples and peaches. Adams and Yang, working at UC Davis, showed convincingly that S-adenosylmethionine (SAM) rather than methionine was a key precursor in ethylene biosynthesis, then in 1979 they topped this triumph by discovering the immediate precursor of ethylene, namely 1-aminocyclopropane-1-carboxylic acid (ACC). Within another few years, Yang and co-workers had managed to define the biochemical pathways that generate ACC from methionine via SAM.
An age of opportunity
Understanding the role of genes involved in ethylene biology creates opportunities for answering many remaining questions about ethylene-driven behaviour. One that remains unresolved concerns regulation of ethylene production in relation to ontogeny of fruit. I observed that tomato fruit harvested less than 15 d after anthesis failed to undergo normal ripening whereas fruit harvested at 20 d or later ripened normally, although with poor eating quality (McGlasson and Adato 1977). How then are ethylene-driven events coordinated with organ ontogeny? There is now a considerable body of evidence on the mode of action of ethylene that will aid such studies.
The discovery of mutant genes from Arabidopsis thaliana plants that are insensitive to ethylene enabled isolation of the ETR1 gene, which encodes an ethylene receptor and is antagonised by competitors of ethylene binding. Similarly, a ripening-impaired mutant tomato (Nr, Never Ripe) has been found to contain a defective homologue of ETR1 that lacks the ability to receive ethylene. The question remains how the different kinds of ethylene receptors might differ in their ethylene response or in their downstream signalling behaviour.
In 1972 we established a distinction between climacteric and non-climacteric fruit in their response to ethylene that led us to propose two systems for regulation of ethylene production. System 1 would be responsible for background ethylene production found in non-climacteric fruit and in pre-climacteric fruit. System 2 would account for the autocatalytic increase in ethylene production associated with ripening in climacteric fruit. Nakatsuka et al. (1998) provided elegant proof for this hypothesis in tomato. Their data suggested that System 1 is mediated by constitutively expressed Le-ACS1A and Le-ACS3 and the negatively feedback-regulated Le-ACS6 (genes encoding ACC synthase enzymes), together with preexisting mRNAs of Le-ACO1 and Le-ACO4 (encoding ACC oxidase proteins that convert ACC to ethylene). In contrast, in System 2 there is a large accumulation of two different ACS mRNAs (Le-ACS2 and Le-ACS4), as well as large increases in Le-ACO1 and Le-ACO4. Similar findings have subsequently been reported for other climacteric fruit. Young developing tomato fruit, while still in System 1, provide excellent experimental material; they behave as a non-climacteric fruit because when treated with propylene, respiration increases temporally without a concomitant increase in endogenous ethylene production. 1-MCP in combination with propylene has turned out to be a very useful tool for distinguishing between events regulated by ethylene and those that are independent (Golding et al. 1998).
The invention of 1-MCP has provided a valuable technology that is widely used to extend the storage life of some fruit, especially several cultivars of apples, and when applied at the right time for each cultivar ensures that the fruit retain good eating quality as well as shelf life. Application of 1-MCP to delay ripening in highly perishable plums can be used to extend shelf life at non-chilling temperatures (<8ºC), leading to a saving in energy costs for refrigeration. The availability of a wide range of new tools that have accompanied the study of ethylene has opened new ways for improving the storage life of fruit that will benefit both domestic and export markets. A current example is the peach, which only has a short cool storage life and responds adversely to treatment with 1-MCP in contrast to the closely related Japanese-type plums that respond beneficially. Furthermore, some late maturing plums have twice the cool storage life of peaches. Imagine the attraction to the consumer if we could transfer these traits from plums to peaches!
References
Adams DO, Yang SF (1979) Ethylene synthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76: 170–174
Blanpied GD (1985) Introduction to the symposium, Ethylene in postharvest biology and technology of horticultural crops. Hort Science 20: 40–41
Golding JB, Shearer D, Wyllie SG, McGlasson WB (1998) Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol Technol 14: 87-98
Lieberman M, Mapson LW (1964) Genesis and biogenesis of ethylene. Nature 204: 343–345
Lyons JM, McGlasson WB, Pratt HK (1962) Ethylene production, respiration, and internal gas concentrations in cantaloupe fruits at various stages of maturity. Plant Physiol 37: 31–36
McGlasson WB, Adato I (1977) Relationship between the capacity to ripen and ontogeny in tomato fruits. Aust J Plant Physiol 4: 451–458
McMurchie EJ, McGlasson WB, Eaks IL (1972) Treatment of fruits with propylene gives information about the biogenesis of ethylene. Nature 237: 235–236
Nakatsuka A, Murachi S, Okunishi H et al. (1998) Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxlate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol 118: 1295-1305
Sisler EC, Serek M (1997) Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant 100: 577–582