By M.D. (Hal) Hatch
Discovering C4 photosynthesis is an instructive story because it says a lot about progress in science, about serendipity, as well as mindsets and our natural resistance to accept results that conflict with the dogma of the day.
As a rule, the major chemical transformations that occur in plants proceed by exactly the same series of steps in all species. For instance, take the process of respiration where sugars and starch are broken down to CO2 and H2O, yielding energy for living cells. It is almost certain that this proceeds by exactly the same 20 or so steps in species right across the Plant Kingdom. In fact, the same process also operates in yeast, mice and man.
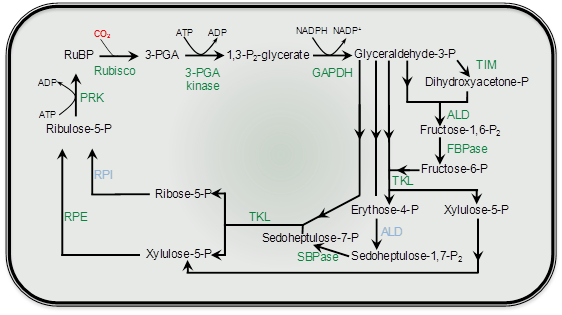
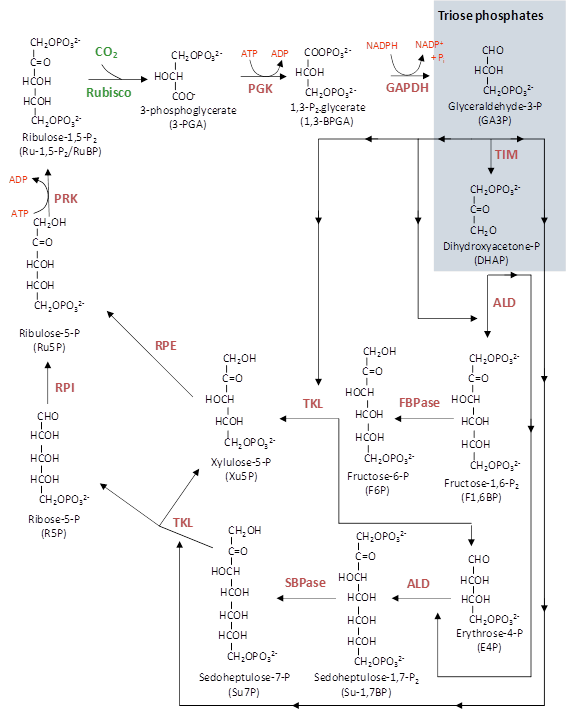
During the 1950s Melvin Calvin and his colleagues at Berkeley resolved the mechanism of photosynthetic CO2 assimilation in the alga Chlorella. Later, they showed that similar steps, with similar enzymes, occurred in a few higher plants. So, by the end of the 1950s it was reasonably assumed that this process, termed the Calvin cycle or photosynthetic carbon reduction (PCR) cycle, accounted for CO2 assimilation in all photosynthetic organisms.
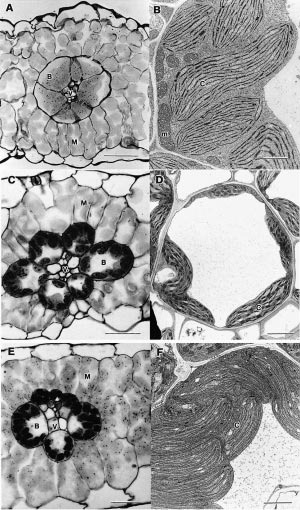
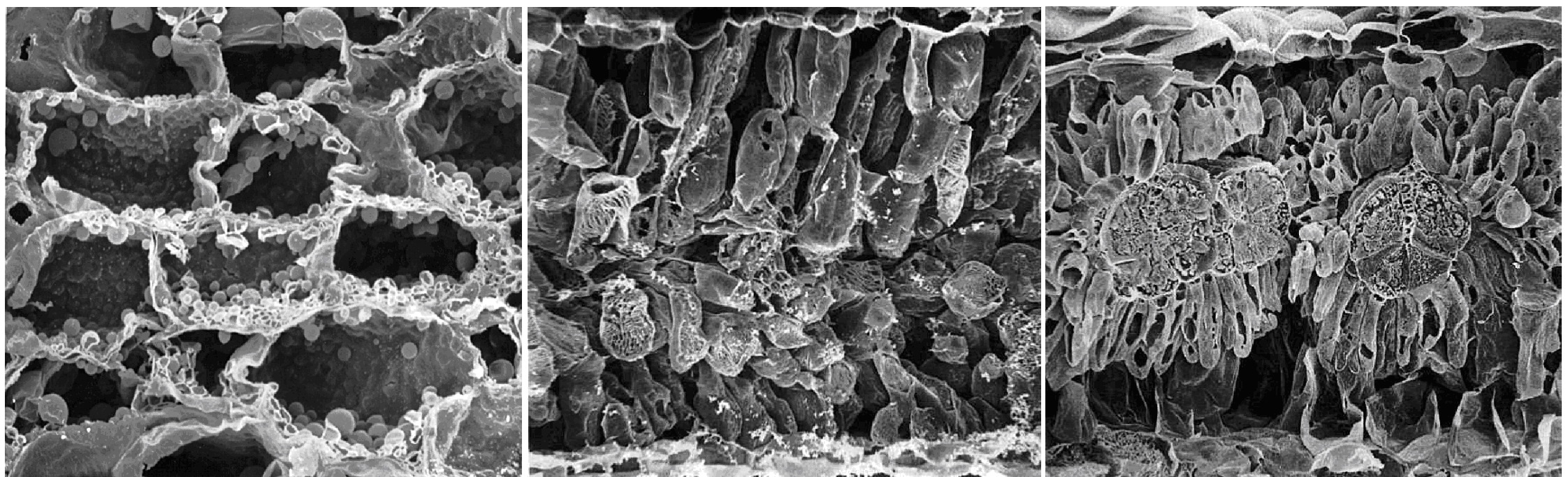
In retrospect, a very observant reader of the plant biological literature of the early 1960s should have noticed that a small group of grass species, including plants like maize, had a set of very unusual but correlated properties, related in one way or another to the process of photosynthesis, that contrasted with the vast majority of other vascular plants. These included an unusual leaf anatomy, substantially higher rates of photosynthesis and growth, higher temperature and light optima for photosynthesis, a much higher water use efficiency, and a very low CO2 compensation point. From this, one might have reasonably concluded that these particular species could be using a different biochemical process for photosynthesis.
We now know that these unusual species fix CO2 by the C4 photosynthetic mechanism. However, the process was not discovered by following up these observations, and only later was the significance of these unusual, correlated features fully appreciated.
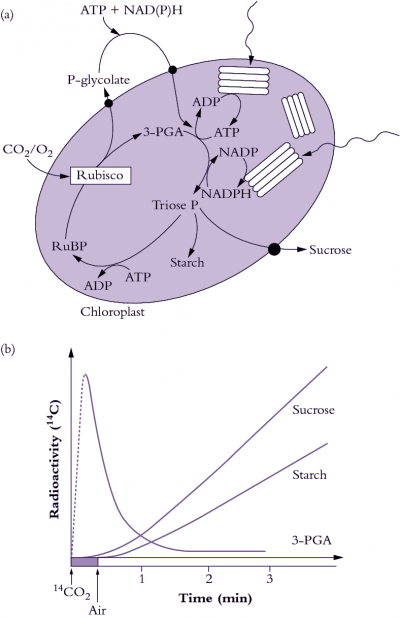
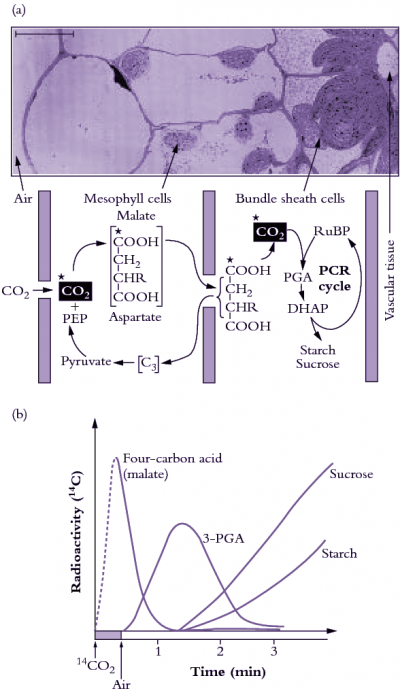
During the early 1960s, my colleague Roger Slack and I were working on aspects of carbohydrate biochemistry and sugar accumulation in sugar cane in the research laboratory of the Colonial Sugar Refining Company in Brisbane. Because of these particular interests, we were in regular contact with a laboratory in Hawaii that also worked on sugar cane. We learned from our Hawaiian colleagues, Hart, Kortschak and Burr, that they had seen some unusual results when they allowed sugar cane leaves to fix radioactive carbon dioxide (14CO2), that is, doing the same experiment that Calvin and his colleagues had done earlier with Chlorella. With this procedure radioactivity should be initially incorporated into the first products formed when CO2 is assimilated; in the case of the PCR cycle the radioactivity should appear in the three-carbon compound 3-phosphoglyceric acid (3-PGA) and then in sugar phosphates. However, when these Hawaiian workers first did this experiment as early as 1957 they saw only minor radiolabelling in 3-PGA after brief exposure to 14CO2 and later they showed that most of the radioactivity was located in the four-carbon dicarboxylic acids, malate and aspartate.
We were really intrigued by this result and had often discussed possible interpretations and significance. So when the Hawaiian group published their results a few years later in 1965 we set about repeating and extending these observations to see if we could find out what it all meant.
Before coming to that work it is worth recounting one other interesting twist to the story. In the late 1960s, and several years after we had begun studying C4 photosynthesis, we became aware of a report published some 10 years earlier in a somewhat obscure annual report of a Russian agricultural research institute. This report from a young Russian scientist, Yuri Karpilov, clearly showed that when maize leaves are exposed to radioactive CO2 most of the radioactivity incorporated after 15 s was not in 3-PGA but was in the same dicarboxylic acids, malate and aspartate, that the Hawaiians had found in labelled sugar cane leaves. In a publication about three years later, Karpilov and a more senior Russian scientist speculated that these results may have been due to faulty killing and extraction procedures. It seems doubtful that they appreciated the full significance of this earlier study.
Our initial experiments were designed to trace the exact fate of carbon assimilated by photosynthesis using 14CO2. Sugar cane leaves were exposed to 14CO2 for various periods under steady-state conditions for photosynthesis, then killed and extracted, and the radioactive products were separated by chromatography, identified and degraded to find out which carbons contained radioactivity. We confirmed the results of the Hawaiian group that most of the radioactivity incorporated after short periods in radioactive CO2 was located in the four-carbon acids malate and aspartate. Substantial radioactive labelling of the PCR cycle intermediates occurred only after longer periods (minutes, rather than seconds).
Critical information was subsequently provided by our so-called ‘pulse–chase’ experiments where a leaf was dosed briefly with 14CO2, and then returned to unlabelled air. The biochemical fate of previously fixed 14C can be followed in sequential samples of tissue. These experiments clearly showed a rapid movement of radioactivity from the four-carbon acid malate into 3-PGA and then later to sugar phosphates and finally into sucrose and starch. There were additional critical results from these initial studies: (1) a chemically unstable dicarboxylic acid, oxaloacetic acid, was rapidly labelled as well as malate and aspartate and was almost certainly the true first product formed; (2) fixed CO2 gave rise to the 4-C carboxyl of these four-carbon acids; and (3) this 4-C carboxyl carbon gave rise to the 1-C carboxyl of 3-PGA. Identification of oxaloacetic acid as an early labelled fixation product was an especially demanding task, and involved generation of a stable derivative that would remain intact during extraction and analysis of 14C fixation products.
Spurred on by this success, we then surveyed a large number of species and found radioactive labelling patterns similar to sugar cane in a number of other grass species, including maize, as well as species from two other plant families. This was an exciting result for us at the time since it clearly showed that this mode of photosynthesis was reasonably widespread taxonomically. The next step in determining the exact nature of this process was to discover the enzymes involved. In species such as sugar cane and maize, there proved to be seven enzyme-catalysed reactions involved in the steps unique to C4 photosynthesis, and these included two steps catalysed by enzymes that had never been described before!
Soon after, we named this process the C4 dicarboxylic acid pathway of photosynthesis — after the first product formed. This was later abbreviated to C4 pathway or C4 photosynthesis and the plants employing this process were termed C4 plants.
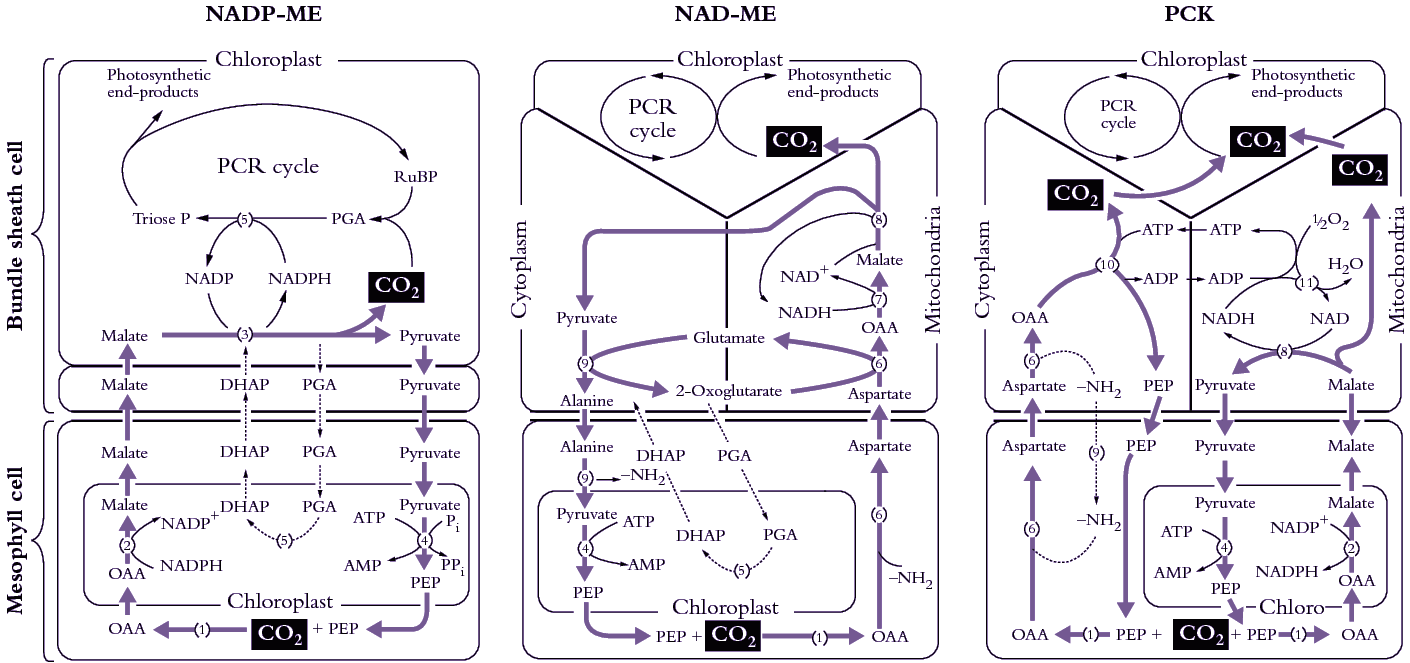
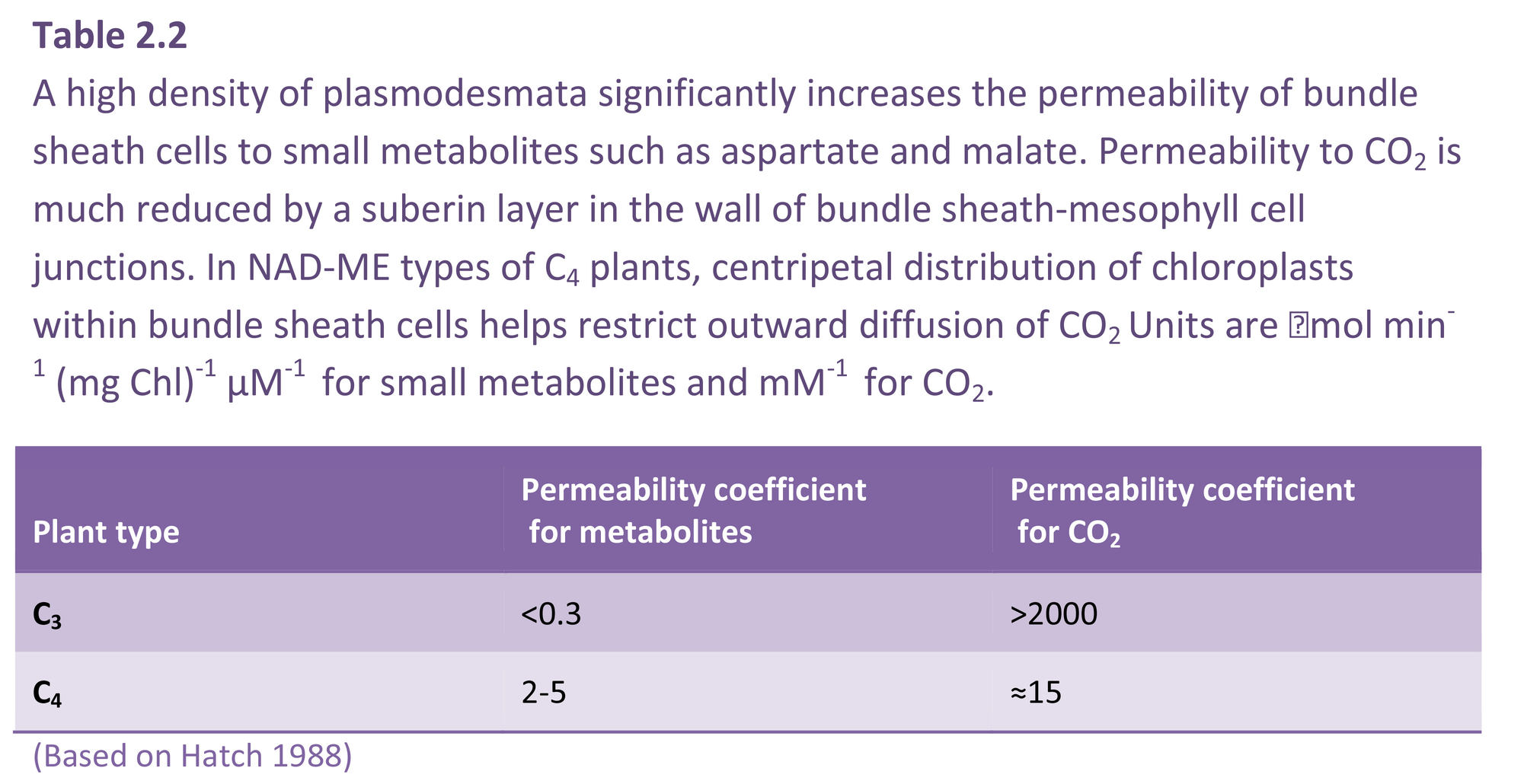
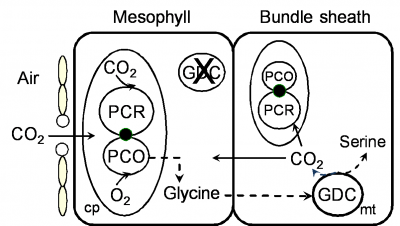
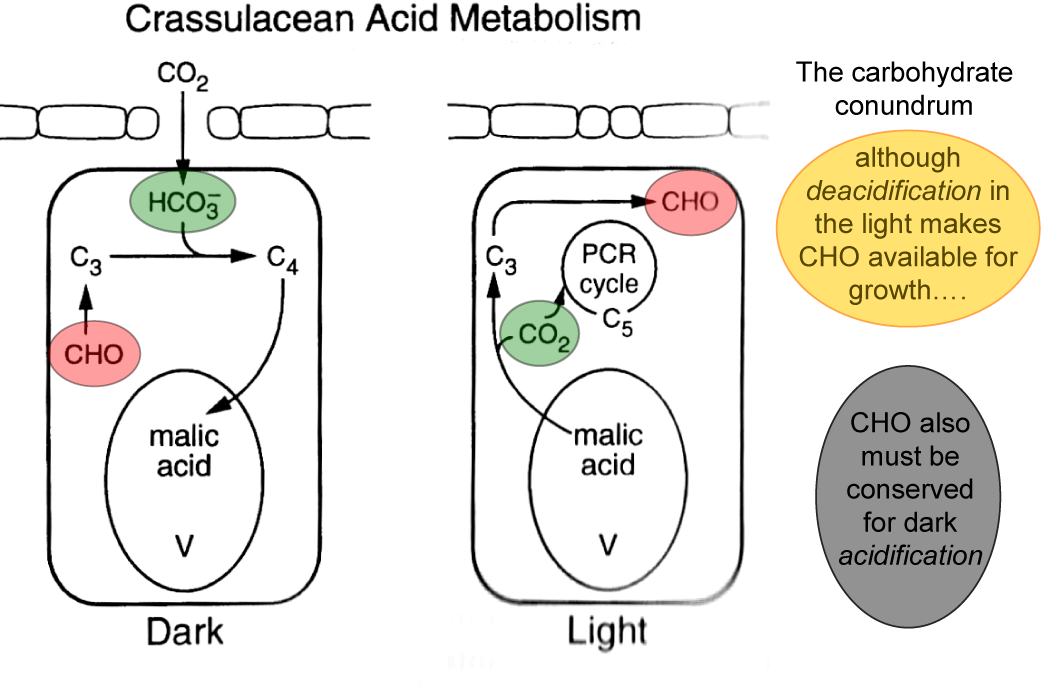
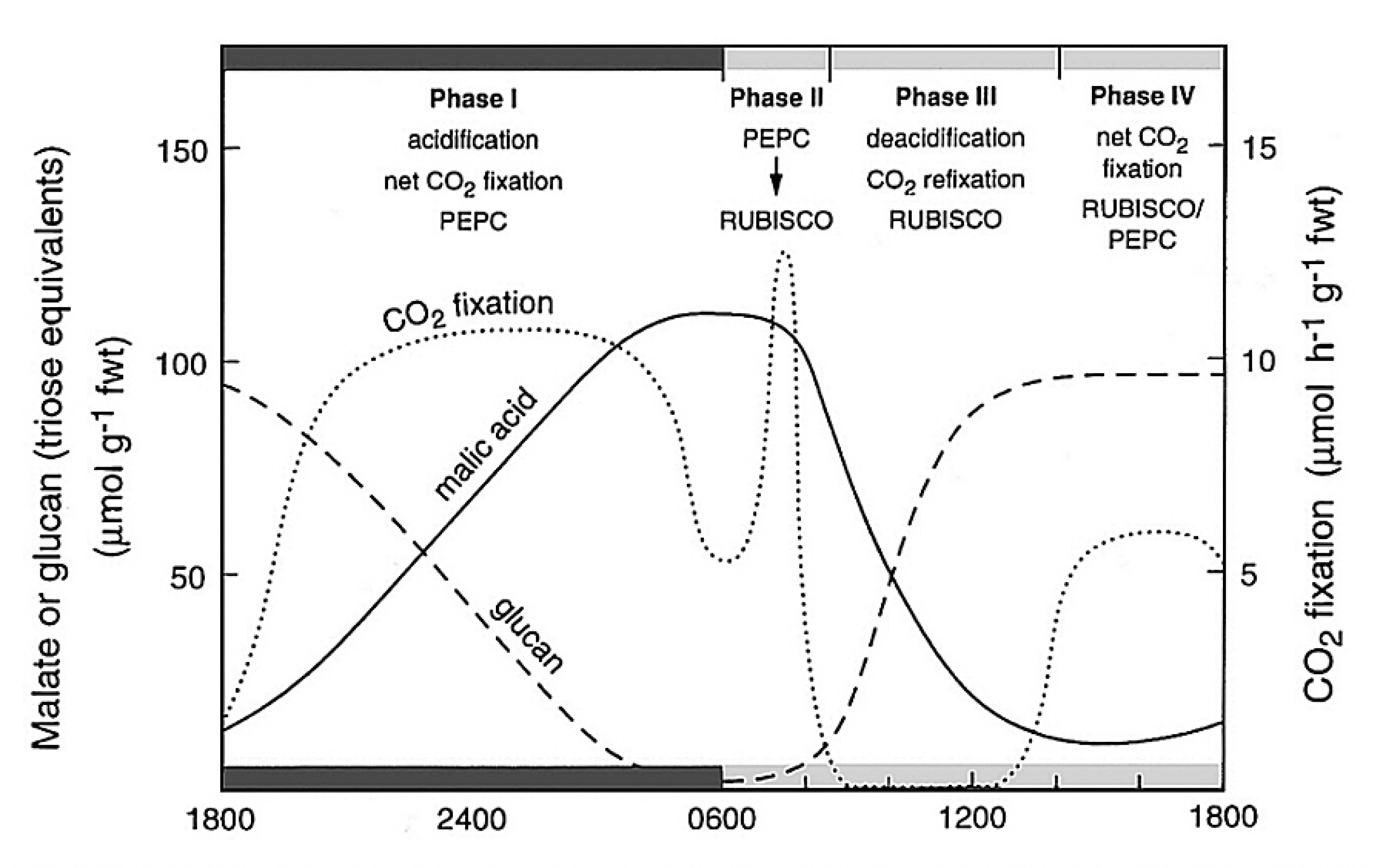
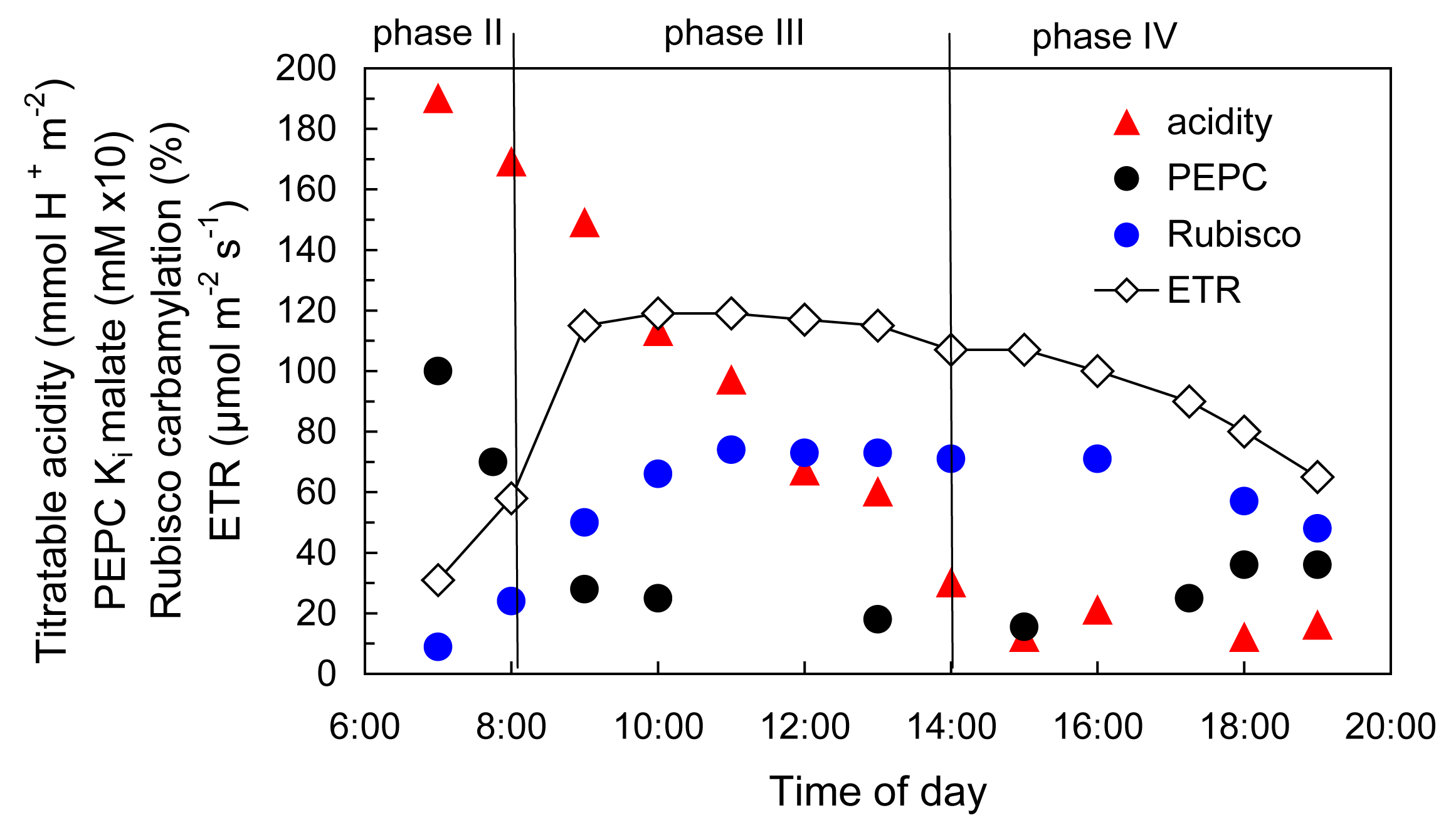
By 1970 we had a reasonably good understanding of how C4 photosynthesis worked in species like maize and sugar cane (see Section 2.2 for details), and suggested that the reactions unique to C4 photosynthesis might function to concentrate CO2 in the bundle sheath cells of C4 leaves, acting essentially as a CO2 pump. Later, we obtained direct experimental evidence that CO2 was indeed concentrated about 10- to 20-fold in these cells in the light.
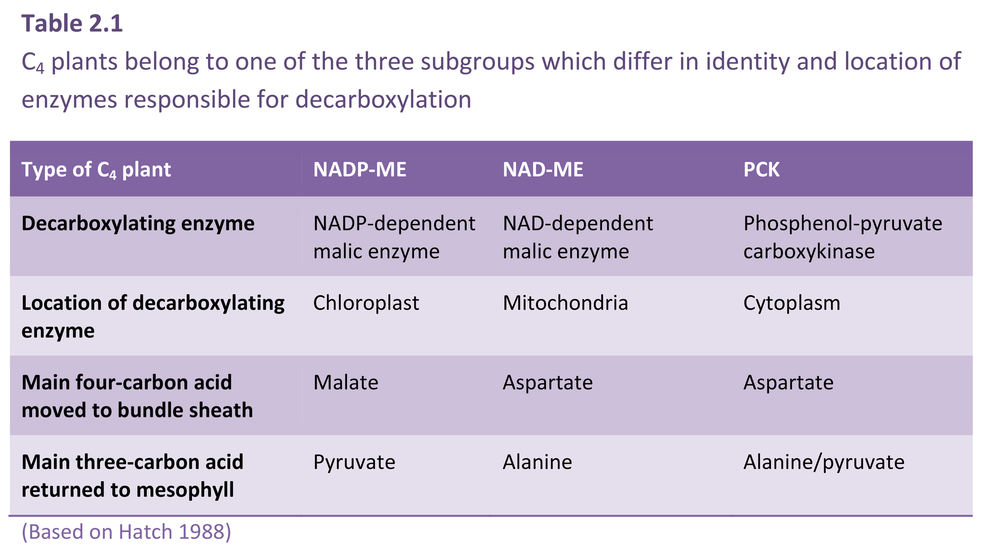
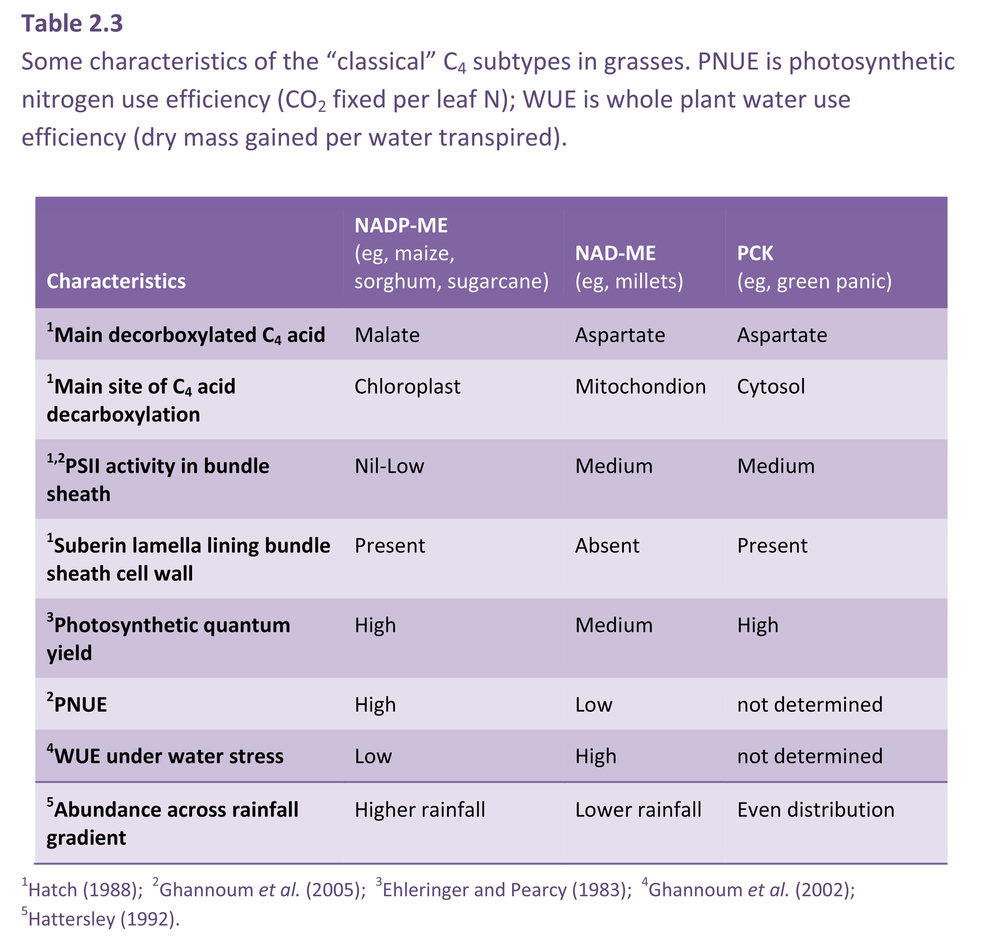
As I mentioned earlier, a major departure from Calvin cycle photosynthesis was never expected. Imagine our surprise, therefore, when it was revealed during the early 1970s that there existed not one, but three different biochemical variants for C4 photosynthesis. On this basis C4 species were divided into three groups, and some connections between process and taxonomic background then emerged.
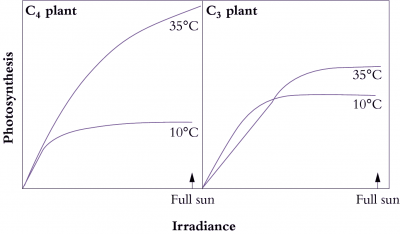
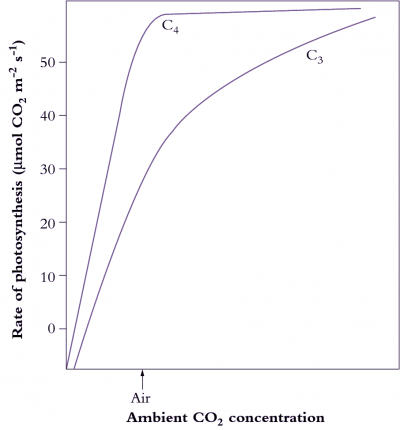
What advantages did all this offer plants over plants that fix CO2 directly by the PCR cycle — that is, using CO2 diffusing directly from air (and distinguished as C3 plants by virtue of their initial three-carbon fixation product phosphoglycerate). As Section 2.2 explains, concentrating CO2 in bundle sheath cells eliminates photorespiration. This, in turn, gives C4 plants distinct advantages in terms of growth and survival, especially at higher temperatures and under strong light. This can be seen most graphically in the distribution of grass species in Australia. In Tasmania, as well as the cooler and wetter southern-most tips of the continent, C4 species are in the minority. However, going north there is a rapid transition and for most of the continent most or all of the grass species are C4.
C4 photosynthesis also offers a potential for growth rates almost twice those seen in C3 plants, but this potential will only be seen at higher temperatures and higher light and this will not be evident in all C4 species. With this kind of growth potential, it is not surprising that C4 species also number among the world’s worst weeds!
As a parting note I should add that about 100 million years ago C3 plants were in their ‘prime’ with atmospheric CO2 concentrations between five and ten times present day levels. However, a new selection pressure then developed. Atmospheric CO2 declined over the next 50–60 million years to something close to our twentieth century levels of about 350 µL–1. This decline almost certainly provided the driving force for evolution of C4 photosynthesis. In other words, C4 photosynthesis was originally ‘discovered’ by nature in the course of overcoming the adverse effects of lower atmospheric CO2 concentration on C3 plants. In effect, C4 processes increase the CO2 concentration in bundle sheath cells to somewhere near the atmospheric CO2 concentration of 100 million years ago.
Further reading
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106
Hatch MD (1992) C4 photosynthesis: an unlikely process full of surprises Plant Cell Physiol 33: 333–342


8b44.jpg?itok=xCTYBDN2)