Fig14.25.png
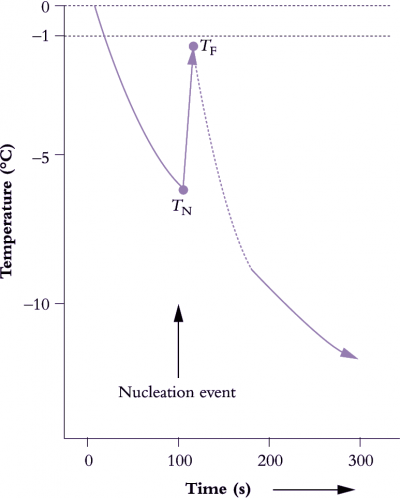
Figure 14.25. A notional cooling curve for an aqueous solution. As heat is extracted steadily, solution temperature falls below 0 °C, and water molecules, now in an unstable state, supercool to around -5 °C. A nucleating event occurs at temperature TN, and heat is released as ice forms (latent heat of fusion), resulting in a sudden increase in temperature to TF. The extent of freezing-point depression (0 – TN °C) also serves as a measure of osmotic pressure. (Original sketch courtesy M.C. Ball)
EW Hewett, Massey University, New Zealand
Ice melts at 0 °C, and an equilibrium mixture of ice and water has traditionally provided a temperature reference for thermocouples. Ice thawing in pure water will maintain a temperature of 0 °C, but if instead of allowing ice to thaw, heat is extracted steadily from a body of water, then ice does not reform at 0 °C (Figure 14.25). Instead, the water will remain liquid, and will supercool until some nucleation event occurs. A tiny particle of ice, or even vibration in the presence of dust particles, is usually sufficient to trigger ice formation together with an abrupt release of heat (latent heat of fusion). In Figure 14.25, TF – TN represents supercooling, and the degree to which TF is less than zero (freezing-point depression) relates to the amount of solute present in solution. Osmotic pressure, vapour-pressure depression and elevation of boiling point are similarly related to the amount of solute present (referred to collectively as colligative properties of solutions).
Highly purified water can be supercooled to about –40 °C, and ice will form spontaneously, but such conditions do not apply in plants because water is not absolutely pure. Instead tissue water is in contact with cell surfaces and invariably holds solutes in solution and colloids in suspension. This aids ice nucleation.