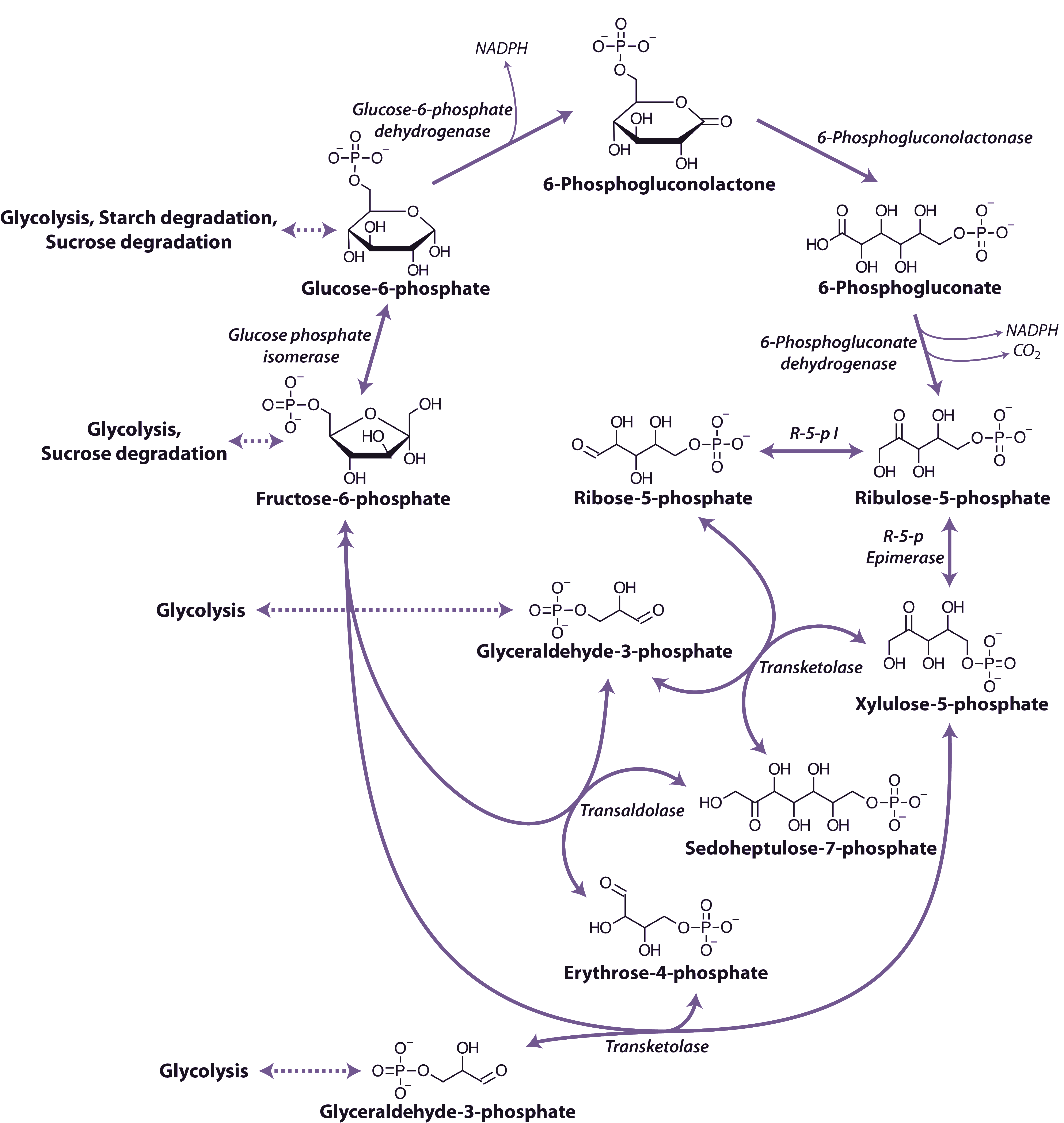
An alternative route for the breakdown of glucose-6-phosphate is provided by the oxidative pentose phosphate pathway (OPP) (Figure 2.22). This pathway functions mainly to generate reductant (i.e. NADPH) for biosynthetic processes including the assimilation of inorganic nitrogen and fatty acid biosynthesis and to maintain redox potential to protect against oxidative stress. In addition, the reversible oxidative section of the pathway is the source of carbon skeletons for the synthesis of a number of compounds. For example ribose-5-phosphate provides the ribosyl moiety of nucleotides and is a precursor for the biosynthesis of purine skeletons and erythrose-4-phosphate, which is the precursor for the biosynthesis of aromatic amino acids by the shikimic acid pathway.
The pathway begins with the dehydrogenation of glucose-6-phosphate catalyzed by glucose-6-phosphate dehydrogenase to produce 6-phosphoglucolactone and is the first step of the oxidative phase of the pathway. The 6-phosphoglucolactone is then hydrolysed to 6-phosphogluconate by 6-phosphogluconolactonase and then undergoes oxidative decarboxylation by 6-phosphogluconate dehydrogenase to produce ribulose-5-phosphate in the final step of the oxidative phase. Overall this phase of the pathway produces two molecules of NADPH from the conversion of glucose-6-phosphate to ribulose-5-phosphate. The non-oxidative phase begins with the reaction of ribulose-5-phosphate with either ribulose-5-phosphate isomerase or ribulose-5-phosphate epimerase followed by a series of reactions catalyzed by transaldolase and transketolase. These reactions result in the production of two molecules of fructose-6-phosphate and one glyceraldehyde 3-phosphate. The glyceraldehyde 3-phosphate and fructose-6-phosphate in the oxidative pentose phosphate pathway may be exchanged with enzymes of glycolysis.
As with glycolysis, reactions of the pentose phosphate pathway are catalysed by different isoforms of the enzymes that occur either in the cytosol or in plastids. Although transketolase and transaldolase may be absent from the cytosol of some species, the activity is maintained by phosphate translocator proteins on the plastid inner-envelope membrane that have the capacity to translocate sugar phosphates.