C4 photosynthesis calls for metabolic compartmentation which is in turn linked to specialised anatomy (Figure 2.4). Three biochemical subtypes of C4 photosynthesis have evolved which probably derive from subtle differences in the original physiology and leaf anatomy of their C3 progenitors.
CO2 assimilation by all three C4 subtypes (Figure 2.8) involves five stages:
- carboxylation of PEP in mesophyll cells, thereby generating four-carbon acids (malate and/or aspartate);
- transport of four-carbon acids to bundle sheath cells;
- decarboxylation of four-carbon acids to liberate CO2;
- re-fixation of this CO2 via Rubisco within the bundle sheath, using the C3 pathway;
- transport of three-carbon acid products following decarboxylation back to mesophyll cells to enable synthesis of more PEP.
Figp2.13.png
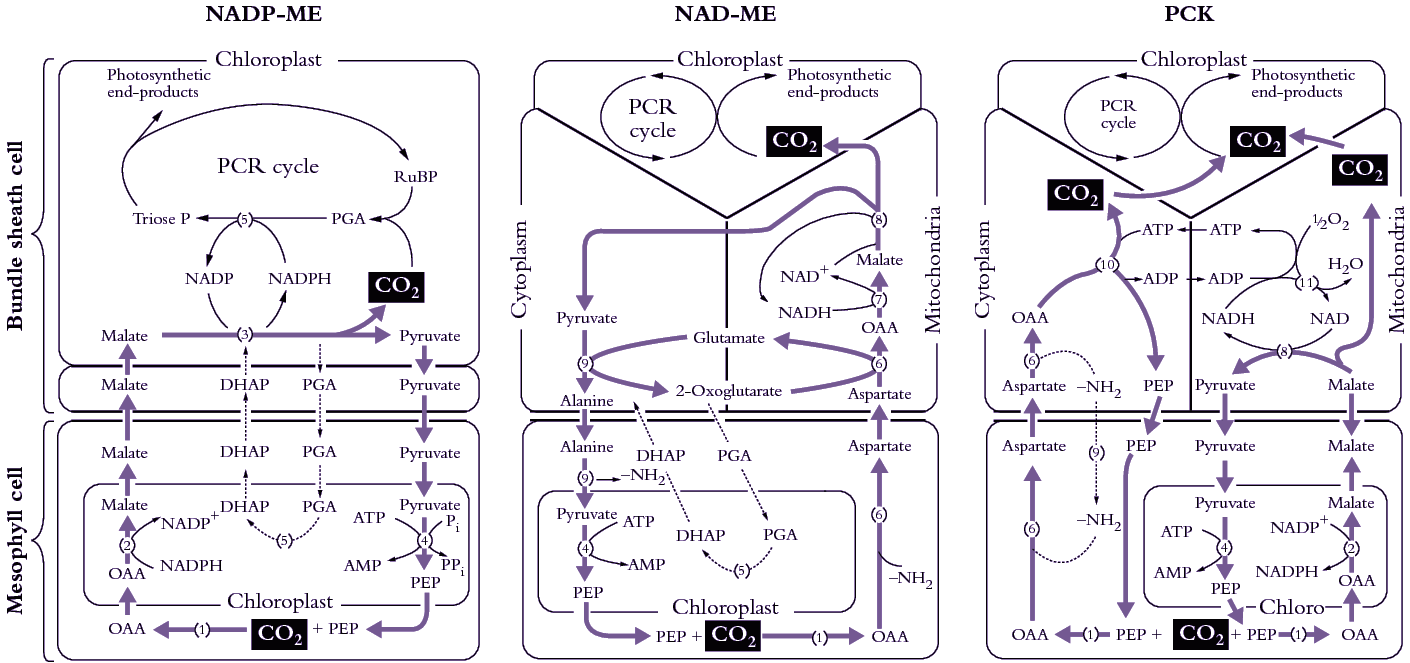
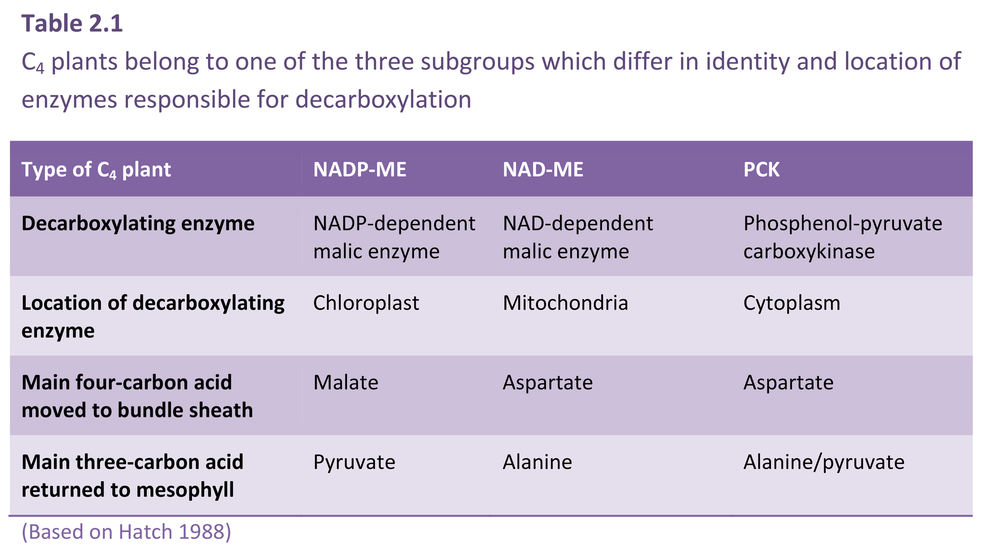
Figure 2.8. C4 plants belong to one of three subtypes represented here (left to right) as NADP-ME, NAD-ME and PCK. Each subtype has a distinctive complement and location of decarboxylating enzymes, and each differs with respect to metabolites transferred between mesophyll and bundle sheath. The path of carbon assimilation and intracellular location of key reactions are shown for each of these biochemically distinct subtypes. Heavy arrows indicate the main path of carbon flow and associated transport of metabolites. Enzymes involved (numbers shown in parentheses) are as follows: (1) PEP carboxylase, (2) NADP-malate dehydrogenase, (3) NADP malic enzyme, (4) pyruvate Pi dikinase, (5) 3-PGA kinase and GAP dehydrogenase, (6) aspartate aminotransferase, (7) NAD-malate dehydrogenase, (8) NAD-malic enzyme, (9) alanine aminotransferase, (10) PEP carboxykinase, (11) mitochondrial NADH oxidation systems. In PCK-type C4 plants, the PGA/DHAP shuttle would also operate between cells as indicated for NADP-ME and NAD-ME. Cycling of anaino groups between mesophyll and bundle sheath cells involves alanine and alanine aminotransferase. (Original diagram courtesy M.D. Hatch).
Recognising some systematic distinctions in whether malate or aspartate was transported to bundle sheath cells, C4 plants were further subdivided into three subtypes according to their four-carbon acid decarboxylating systems and ultrastructural features (Hatch et al. 1975). Members of each subtype contain high levels of either NADP-malic enzyme (NADP-ME), phosphoenolpyruvate carboxykinase (PCK) or NAD-malic enzyme (NAD-ME) (so designated in Figure 2.8). High NADP-malic enzyme activity is always associated with higher NADP-malate dehydrogenase activity, while those species featuring high activities of either of the other two decarboxylases always contain high levels of aminotransferase and alanine aminotransferase activities. As a further distinction, each of the decarboxylating enzymes is located in bundle sheath cells; NAD-malic enzyme is located in mitochondria but PEP carboxykinase is not.
In all three subtypes, the primary carboxylation event occurs in mesophyll cytoplasm with PEP carboxylase acting on HCO3– to form oxaloacetate. However, the fate of this oxaloacetate varies according to subtype (Table 2.1; Figure 2.8). In NADP-ME species, oxaloacetate is quickly reduced to malate in mesophyll chloroplasts using NADPH. By contrast, in NAD-ME and PCK species, oxaloacetate is transaminated in the cytoplasm, with glutamate donating the amino group, to generate aspartate. Thus, malate is transferred to bundle sheath cells in NADP-ME species and aspartate is transferred in NAD-ME and PCK species. The chemical identity of three-carbon acids returned to mesophyll cells varies accordingly.
In NADP-ME species, only chloroplasts are involved in decarboxylation and subsequent carboxylation via the PCR cycle (Figure 2.8). By contrast, in NAD-ME and PCK species, chloroplasts, cytoplasm and mitochondria are all involved in moving carbon to the PCR cycle of bundle sheath chloroplasts. In NAD-ME and PCK species, aspartate arriving in bundle sheath cells is reconverted to oxaloacetate in either mitochondria (NAD-ME) or cytoplasm (PCK) (Table 2.2). Reduction and decarboxylation of oxaloacetate occurs in mitochondria of NAD-ME species and CO2 is thereby released for fixation by chloroplasts of bundle sheath cells. In PCK species, oxaloacetate in the cytoplasm is decarboxylated by PCK, thereby releasing CO2 for fixation in bundle sheath chloroplasts (Figure 2.8).
Transport of metabolites to bundle sheath cells
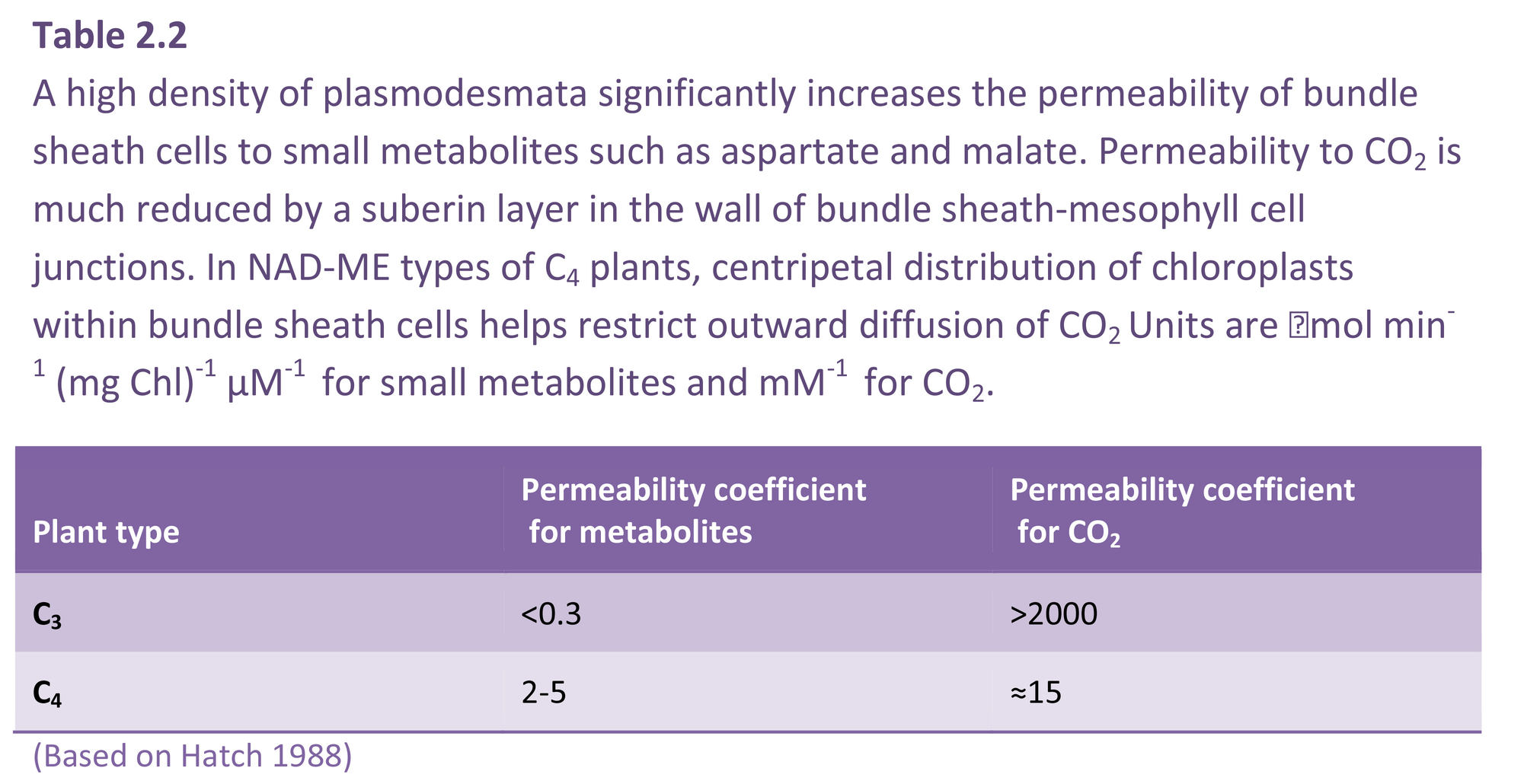
A rapid transfer of malate and aspartate to bundle sheath cells from mesophyll cells is required if the CO2 concentration in bundle sheath cells is to stay high. A very high density of plasmodesmata linking bundle sheath cells to mesophyll cells facilitates this traffic. Consequently, the permeability coefficient of C4 bundle sheath cells to small metabolites such as four-carbon acids is about 10 times larger than that of C3 mesophyll cells (Table 2.2). However, coupled with this need for a high permeability to metabolites moving into bundle sheath cells is a low permeability to CO2 molecules so that CO2 released through decarboxylation in the bundle sheath does not diffuse rapidly into mesophyll air spaces. For some species, a layer of suberin in the cell wall of bundle sheath–mesophyll junctions (suberin lamella) significantly reduces CO2 efflux (Table 2.2).
Centrifugal versus centripetal chloroplasts
Not all species contain a suberin layer, but all C4 plants have a need to prevent CO2 from diffusing quickly out of bundle sheath cells, so that the location of chloroplasts of bundle sheath cells becomes critical in those species lacking a suberin layer (Figure 2.9). Where species have a suberin layer, chloroplasts are located in a centrifugal position, that is, on the wall furtherest away from the centre of the vascular bundle lying in the middle of the bundle sheath (Figure 2.9E, F). In those C4 species lacking a suberin layer, chloroplasts are located centripetally, that is, on the wall closest to the centre of the vascular bundle lying within the bundle sheath (Figure 2.9A, B). Such a location would help restrict CO2 diffusion from bundle sheath to mesophyll cells.
Fig 2.14.jpg
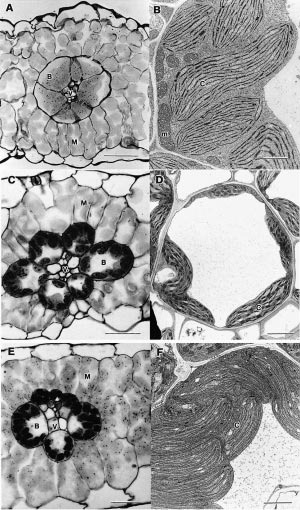
Figure 2.9. C4 plants belong to one of three subtypes shown here in cross-section as light micrographs (left side) and electron micrographs (right side). Top to bottom, these subtypes are designated NAD-ME (A, B) PCK (C, D) and NADP-ME (E, F). Features common to all subtypes include a vascular bundle (V), bundle sheath (B), mesophyll tissue (M) and chloroplasts (C).
Subtype NAD-ME (A, B) is represented by Amaranthus edulis and shows bundle sheath cells with centripetally located chloroplasts containing small starch grains and surrounded by mesophyll cells. The accompanying electron micrograph of a cytoplasmic region of a bundle sheath cell shows chloroplasts and numerous large mitochondria. Scale bar in A = 50 µm; in B = 2 µm.
Subtype PCK (C, D) is represented by Chloris gayana with chloroplasts arranged around the periphery of bundle sheath cells and adopting a centrifugal position. Mitochondria show well-developed internal membrane structures. Scale bar in C = 25 µm; in D = 3 µm.
Subtype NADP-ME is represented by Zea mays where the bundle sheath contains centrifugally located chloroplasts with numerous starch grains, but lacking grana. Chloroplasts in adjacent mesophyll cells are strongly granal. Bundle sheath cells contain few mitochondria and these show only moderate development of internal membrane structures. Scale bar in E = 25 µm; in F scale bar = 2 µm (Micrographs courtesy Stuart Craig and Celia Miller).
Regulation of C4 photosynthesis
Fixation of CO2 by C4 plants involves the coordinated activity of two cycles in separate anatomical compartments (Figure 2.8). The first cycle is C4 (carboxylation by PEP carboxylase), the second is C3 (carboxylation by Rubisco). Given this biochemical and anatomical complexity, close regulation of enzyme activities is a prerequisite for efficient coordination.
PEP carboxylase, NADP-malate dehydrogenase and pyruvate orthophosphate dikinase are all light-regulated and their activities vary according to irradiance. NADP-malate dehydrogenase is regulated indirectly by light via the thioredoxin system.
PEP carboxylase in C4 plants exists in the same homo-tetramer in light- and dark-acclimated leaves. This is in marked contrast to CAM species where different forms exist in light- and dark-acclimated leaves. In C4 plants, PEP carboxylase has extremely low activity at night, thus preventing uncontrolled consumption of PEP. Such complete loss of activity in darkness is mediated via divalent metal ions, pH plus allosteric activators and inhibitors. As a consequence, and over a period of days, C4 plants can increase or decrease PEP carboxylase in response to light regime.