2.0-Ch-Fig-2.35.png
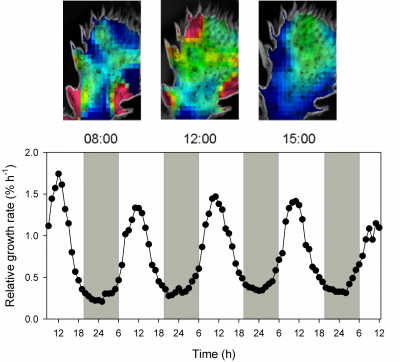
Figure 2.35 Diel expansion growth of Opuntia oricola cladodes during drought in the desert biome of the Biosphere 2 Laboratory in Oracle Arizona USA measured by time-lapse photography. Heterogeneity of growth rate throughout the day is colour coded as red = 2.0% to blue = 0.5% per hour (Diagram by B. Osmond based on Gouws et al. Funct Plant Biol 32: 421-428, 2005)
Compared to the photosynthetic biochemistry and physiology in leaves of C3 and C4 plants, the 6% of taxa estimated to exhibit CAM (in at least 35 families and >400 genera) express it with staggering variety (Winter et al. 2015). That is, the distinctive biochemical attributes of CAM outlined above, derived from a handful of research-compliant leafy model species, are but the tip of an iceberg of what really qualifies as a CAM plant (Borland et al. 2011).
The following summary of some distinctive physiological attributes of CAM underscores this conundrum:
Biochemical and physiological determinants of stable isotopic composition of plants with CAM. Fixation of CO2 by PEPC and Rubisco in vitro show clearly different discriminations against the heavier, naturally occurring, non-radioactive (stable) 13C isotope of carbon when expressed as a \(\delta\)13C value. Thus total carbon in C4 plants reflects a small discrimination against 13C resulting in \(\delta\)13C values of about –12.5 ‰, with more negative values in C3 plants (about –27 ‰). It is therefore not surprising that CAM plants tend to fall between these values depending on the balance between total carbon assimilated by PEPC in phase I and that added by Rubisco in phase IV. Partial closure of stomata adds a diffusional discrimination to the biochemical discrimination associated with Rubisco, so \(\delta\)13C values in C3 plants (and CAM plants) become less negative under water stress (Griffiths et al. 2007). Recently it has been suggested that unequivocal identification of CAM can be assigned on the basis of net nocturnal CO2 assimilation, acidification and \(\delta\)13C values less negative than -20 ‰. If some dark CO2 uptake and net acidification is detectable, but \(\delta\)13C is more negative than -20 ‰, these plants would be designated as C3-CAM species, indicating that CAM is present but the contribution of the CAM pathway to net 24h carbon gain is small in comparison to the contribution of daytime CO2 uptake (Winter et al. 2015).
- Stomatal opening in the dark is a fundamental physiological feature of CAM. Dark CO2 fixation lowers internal [CO2] and promotes stomatal opening. Stomata retain their responsiveness to external CO2 in phases I and IV, opening further when external CO2 is reduced. They do not respond to low external CO2 during phase III, when closure occurs in response to high internal [CO2] from deacidification of malic acid but do seem to sense the completion of deacidification itself.
- CAM is essentially a single cell phenomenon and succulence (low surface to volume ratio) is a feature of many but not all CAM plants. Succulence makes at least two important contributions to the physiology of CAM: large vacuoles for malic acid storage in mesophyll cells and tight packing of cells with small intercellular spaces. The latter means that CO2 diffusion internally is largely confined to wet cell walls and is thus 3 to 4 orders of magnitude slower than in the gas phase, potentially mitigating CO2 fixation by Rubisco in phase IV. It remains to be seen whether Clusia rosea may have resolved this trade-off by anatomical/physiological differentiation. Enlarged, tightly-packed PEPC-enriched upper palisade cells have a potential for nocturnal CO2 fixation and acidification whereas lower spongy mesophyll cells exhibit predominantly C3 metabolism (Zambrano et al. 2014).
- Whereas leaf expansion growth of C3 plants in the hot dry desert usually occurs at night, leaves and cladodes of some CAM plants grow in the light during phase III (Figure 2.35). This is not surprising because this phase coincides with reliable availability of CHO, maximum temperature and highest cytoplasmic acidity required for growth (Gouws et al. 2005). On the other hand Mesembryanthemum crystallinum (a facultative CAM plant) shows maximum growth in the dark.
